Trends in Real-World Neovascular AMD Treatment Outcomes in the UK
- PMID: 33116384
- PMCID: PMC7569079
- DOI: 10.2147/OPTH.S275977
Trends in Real-World Neovascular AMD Treatment Outcomes in the UK
Abstract
Purpose: To report trends in real-world outcomes of anti-vascular endothelial growth factor (anti-VEGF) therapy for neovascular age-related macular degeneration (nAMD) in the United Kingdom (UK) over the last decade.
Design: Systematic review.
Methods: Medline, PubMed, and Embase databases were searched from 9 April 2010 to 8 April 2020 for publications that met the inclusion criteria: treatment-naïve eyes, UK-only data and ≥1 year of follow-up. ICHOM (International Consortium for Health Outcome Measures) outcomes and study quality were assessed. Visual acuity (VA) trends were assessed in studies with ≥100 eyes at baseline.
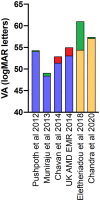
Results: Twenty-six studies (n=25,761 eyes) were included, meeting 14-17 out of 20 Institute of Health Economics Quality Appraisal of Case Series checklist domains. Only ranibizumab and aflibercept outcome data were available. The mean injection number in the first year of treatment was 5.9 in publications from 2010 to 2015 and 7.1 from 2015 to 2020. Average baseline VA and mean one-year, two-year and three-year VA gains gradually improved over the last decade. Longer-term studies reported that the visual gains achieved in the first year of treatment were rarely maintained, with under-treatment a likely contributing factor.
Conclusion: UK real-world outcomes have improved over the last decade with improved service delivery and the adoption of more proactive treatment regimens but are still not always as impressive as registration clinical trial results. Access to longer-acting anti-VEGF therapies would reduce the treatment burden for patients, carers, and the healthcare system, potentially making replication of clinical trial results possible in the NHS.
Keywords: aflibercept; anti-VEGF; anti-vascular endothelial growth factor; intravitreal therapy; macular degeneration; ranibizumab; systematic review; treatment.
© 2020 Mehta et al.
Conflict of interest statement
HM has received research grants, educational travel grants and honoraria from Allergan/AbbVie, Bayer, Novartis and Roche outside the submitted work. TM is a consultant for Allergan, Bayer and Novartis; and reports grants, personal fees, non-financialsupport from Novartis and non-financial support from Bayer outside the submitted work. FG has received honoraria for consultancy-advisory boards from Alimera, Allergan, Bayer, Novartis, Oxford BioElectronics, Roche; educational travel grants from Allergan, Bayer, Novartis and departmental research grants from Allergan, Bayer, Boehringer Ingelheim, Chengdu Pharma, Novartis, PanOptica and Roche; and reports grants, personal fees from Allergan, grants from Bayer, personal fees from Novartis, outside the submitted work. WMA has received honoraria for advisory board memberships from AbbVie, Alcon, Alimera, Allergan, Bayer, Bausch and Lomb, Novartis and Pfizer, speaker Fees from Alimera, Allergan, Bayer, Novartis and Pfizer, and educational travel grants from Alimera, Allergan, Bayer, Novartis and Pfizer; and reports grants and personal fees from Novartis, Bayer and Allergan, personal fees from Roche, AbbVie, and Alimera, and grants from Boehringer Ingelheim, outside the submitted work. WMA has undertaken clinical research sponsored by Allergan, Bayer, Gyroscope, and Novartis and his institution has received research funding from Allergan, Bayer, Boehringer Ingelheim, CenterVue, Novartis, and Optos plc. LK is a consultant for AbbVie, Alcon, Allergan, Bayer, Kris, Novartis and Théa laboratories; and reports personal fees from Bayer, grants from Allergan, outside the submitted work. LNK and PZ have no disclosures and the authors report no other potential conflicts of interest for this work.
Figures


References
-
- Li JQW, Schmid M, Letow J, Wolpers AC, Holz FG, Finger RP. EURETINA. Retinal Diseases in Europe: Prevalence. Incidence and Healthcare Needs. Bonn, Germany; 2017. Available from: https://www.euretina.org/downloads/EURETINA_Retinal_Diseases.pdf. Accessed September30, 2020.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

