Turbulent Systolic Flow Downstream of a Bioprosthetic Aortic Valve: Velocity Spectra, Wall Shear Stresses, and Turbulent Dissipation Rates
- PMID: 33117194
- PMCID: PMC7550765
- DOI: 10.3389/fphys.2020.577188
Turbulent Systolic Flow Downstream of a Bioprosthetic Aortic Valve: Velocity Spectra, Wall Shear Stresses, and Turbulent Dissipation Rates
Abstract
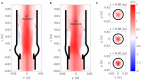
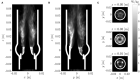
Every year, a quarter million patients receive prosthetic heart valves in aortic valve replacement therapy. Prosthetic heart valves are known to lead to turbulent blood flow. This turbulent flow field may have adverse effects on blood itself, on the aortic wall and on the valve performance. A detailed characterization of the turbulent flow downstream of a valve could yield better understanding of its effect on shear-induced thrombocyte activation, unphysiological wall shear stresses and hemodynamic valve performance. Therefore, computational simulations of the flow past a bioprosthetic heart valve were performed. The computational results were validated against experimental measurements of the turbulent flow field with tomographic particle image velocimetry. The turbulent flow was analyzed for disturbance amplitudes, dissipation rates and shear stress distributions. It was found that approximately 26% of the hydrodynamic resistance of the valve was due to turbulent dissipation and that this dissipation mainly took place in a region about one valve diameter downstream of the valve orifice. Farther downstream, the turbulent fluctuations became weaker which was also reflected in the turbulent velocity spectra of the flow field. Viscous shear stresses were found to be in the range of the critical level for blood platelet activation. The turbulent flow led to elevated shear stress levels along the wall of the ascending aorta with strongly fluctuating and chaotic wall shear stress patterns. Further, we identified leaflet fluttering at 40 Hz which was connected to repeated shedding of vortex rings that appeared to feed the turbulent flow downstream of the valve.
Keywords: bioprosthetic heart valve; computational fluid dynamics; fluid-structure interaction; laminar-turbulent transition; leaflet fluttering; thrombocyte activation; wall shear stress.
Copyright © 2020 Becsek, Pietrasanta and Obrist.
Figures









References
-
- Armani D., Liu C., Aluru N. (1999). Re-configurable fluid circuits by pdms elastomer micromachining, in Technical Digest. IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 99CH36291) (Orlando, FL: IEEE; ), 222–227.
-
- Betts J. G, Young K. A., Wise J. A., Johnson E., Poe B., Kruse D. H., et al. (2013). Anatomy and Physiology. Houston, TX: OpenStax; Available online at: https://openstax.org/books/anatomy-and-physiology/pages/1-introduction
LinkOut - more resources
Full Text Sources
Miscellaneous

