Analysis of the Cell Type-Dependence on the Arenavirus Z-Mediated Virus-Like Particle Production
- PMID: 33117310
- PMCID: PMC7561441
- DOI: 10.3389/fmicb.2020.562814
Analysis of the Cell Type-Dependence on the Arenavirus Z-Mediated Virus-Like Particle Production
Abstract
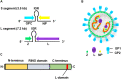
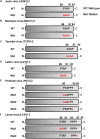
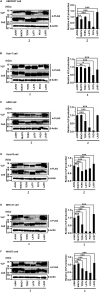
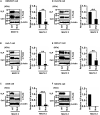
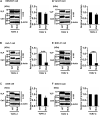
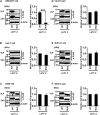
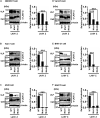
Several arenaviruses are highly pathogenic to humans, causing hemorrhagic fever. Discovery of anti-arenavirus drug candidates is urgently needed, although the molecular basis of the host- and organ-specific pathogenicity remains to be fully elucidated. The arenavirus Z protein facilitates production of virus-like particles (VLPs), providing an established method to assess virus budding. In this study, we examined the efficiency of VLP production by solely expressing Z protein of several different arenaviruses. In addition, we analyzed the role of the late (L)-domain of the arenavirus Z protein, which is essential for the interaction with ESCRT proteins, in VLP production among different cell lines. VLP assay was performed using Z proteins of Junín virus (JUNV), Machupo virus (MACV), Tacaribe virus (TCRV), Latino virus (LATV), Pichinde virus (PICV), and Lassa virus (LASV) in six different cell lines: HEK293T, Huh-7, A549, Vero76, BHK-21, and NIH3T3 cells. JUNV, MACV, and LASV Z proteins efficiently produced VLPs in all tested cell lines, while the efficiencies of VLP production by the other arenavirus Z proteins were cell type-dependent. The contribution of the L-domain(s) within Z protein to VLP production also highly depended on the cell type. These results suggested that each arenavirus has its own particle-production mechanism, which is different among the cell types.
Keywords: L-domain; Z; arenavirus; cell type-dependence; virus-like particle.
Copyright © 2020 Mpingabo, Urata and Yasuda.
Figures


References
-
- Buchmeier M. J., Peters C. J., de la Torre J. C. (2007). Arenaviridae: the virus and their replication. Fiel. Virol. 2 1792–1827.
LinkOut - more resources
Full Text Sources

