Advances in hepatitis B therapeutics
- PMID: 33117536
- PMCID: PMC7570774
- DOI: 10.1177/2049936120965027
Advances in hepatitis B therapeutics
Abstract
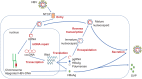
Despite the availability of both effective preventive vaccines and oral antivirals, over 250 million people are chronically infected with the hepatitis B virus (HBV). Globally, chronic hepatitis B is the leading cause of hepatocellular carcinoma, which represents the third cause of cancer mortality, accounting for nearly 1 million annual deaths. Current oral nucleos(t)ide therapy with tenofovir or entecavir suppresses serum HBV-DNA in most treated patients, but rarely is accompanied by HBsAg loss. Thus, treatment has to be given lifelong to prevent viral rebound. A broad spectrum of antivirals that block the HBV life cycle at different steps are in clinical development, including entry inhibitors, cccDNA disrupters/silencers, translation inhibitors, capsid assembly modulators, polymerase inhibitors and secretion inhibitors. Some of them exhibit higher potency than current oral nucleos(t)ides. Drugs in more advanced stages of clinical development are bulevirtide, JNJ-6379, ABI-H0731, ARO-HBV and REP-2139. To date, only treatment with ARO-HBV and with REP-2139 have resulted in HBsAg loss in a significant proportion of patients. Combination therapies using distinct antivirals and/or immune modulators are expected to maximize treatment benefits. The current goal is to achieve a 'functional cure', with sustained serum HBsAg after drug discontinuation. Ultimately, the goal of HBV therapy will be virus eradication, an achievement that would require the elimination of the cccDNA reservoir within infected hepatocytes.
Keywords: antiviral therapy; bulevirtide; cccDNA; chronic hepatitis B; combination therapy; gene editing; hepatitis delta; resistance.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest: The authors declare that there is no conflict of interest.
Figures



References
-
- Tang L, Covert E, Wilson E, et al. Chronic hepatitis B infection: a review. JAMA 2018; 319: 1802–1813. - PubMed
-
- World Health Organization (WHO). Hepatitis B [Internet]. https://www.who.int/es/news-room/fact-sheets/detail/hepatitis-b (2019, accessed 15 July 2020).
-
- Cornberg M, Lok A, Terrault N, et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B – Report from the 2019 EASL-AASLD HBV treatment endpoints conference. Hepatology 2020; 72: 539–557. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

