The Role of 18F-FDG PET/CT in Guiding Precision Medicine for Invasive Bladder Carcinoma
- PMID: 33117695
- PMCID: PMC7574640
- DOI: 10.3389/fonc.2020.565086
The Role of 18F-FDG PET/CT in Guiding Precision Medicine for Invasive Bladder Carcinoma
Abstract
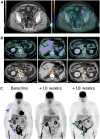
Bladder cancer (BC) is the 10th most common cancer worldwide. Approximately one quarter of patients with BC have muscle-invasive disease (MIBC). Muscle-invasive disease carries a poor prognosis and choosing the optimal treatment option is critical to improve patients' outcomes. Ongoing research supports the role of 2-deoxy-2-(18F)fluoro-D-glucose positron emission tomography (18F-FDG PET) in guiding patient-specific management decisions throughout the course of MIBC. As an imaging modality, 18F-FDG PET is acquired simultaneously with either computed tomography (CT) or MRI to offer a hybrid approach combining anatomical and metabolic information that complement each other. At initial staging, 18F-FDG PET/CT enhances the detection of extravesical disease, particularly in patients classified as oligometastatic by conventional imaging. 18F-FDG PET/CT has value in monitoring response to neoadjuvant and systemic chemotherapy, as well as in localizing relapse after treatment. In the new era of immunotherapy, 18F-FDG PET/CT may also be useful to monitor treatment efficacy as well as to detect immune-related adverse events. With the advent of artificial intelligence techniques such as radiomics and deep learning, these hybrid medical images can be mined for quantitative data, providing incremental value over current standard-of-care clinical and biological data. This approach has the potential to produce a major paradigm shift toward data-driven precision medicine with the ultimate goal of personalized medicine. In this review, we highlight current literature reporting the role of 18F-FDG PET in supporting personalized management decisions for patients with MIBC. Specific topics reviewed include the incremental value of 18F-FDG PET in prognostication, pre-operative planning, response assessment, prediction of recurrence, and diagnosing drug toxicity.
Keywords: PET – Positron Emission Tomography; bladder cancer; immunotherapy; muscle invasive bladder cancer; staging.
Copyright © 2020 Girard, Vila Reyes, Shaish, Grellier, Dercle, Salaün, Delcroix and Rouanne.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

