Lasso Peptides: Heterologous Production and Potential Medical Application
- PMID: 33117783
- PMCID: PMC7549694
- DOI: 10.3389/fbioe.2020.571165
Lasso Peptides: Heterologous Production and Potential Medical Application
Abstract
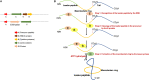
Lasso peptides are natural products found in bacteria. They belong to a specific family of ribosomally-synthesized and posttranslationally-modified peptides with an unusual lasso structure. Lasso peptides possess remarkable thermal and proteolytic stability and various biological activities, such as antimicrobial activity, enzyme inhibition, receptor blocking, anticancer properties and HIV antagonism. They have promising potential therapeutic effects on gastrointestinal diseases, tuberculosis, Alzheimer's disease, cardiovascular disease, fungal infections and cancer. Lasso peptides with high stability have been shown to be good carriers for other bioactive peptides. These make them attractive candidates for pharmaceutical research. This review aimed to describe the strategies used for the heterologous production of lasso peptides. Also, it indicated their therapeutical potential and their capacity to use as an efficient scaffold for epitope grafting.
Keywords: bioactivity; biosynthesis; heterologous expression; lasso peptides; medical application.
Copyright © 2020 Cheng and Hua.
Figures
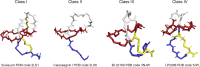


References
-
- Arnison P. G., Bibb M. J., Bierbaum G., Bowers A. A., Bugni T. S., Bulaj G., et al. (2013). Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30 108–160. 10.1039/c2np20085f - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

