Knockdown of FOXA2 Impairs Hair-Inductive Activity of Cultured Human Follicular Keratinocytes
- PMID: 33117803
- PMCID: PMC7578224
- DOI: 10.3389/fcell.2020.575382
Knockdown of FOXA2 Impairs Hair-Inductive Activity of Cultured Human Follicular Keratinocytes
Abstract
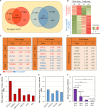
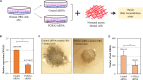
Reciprocal interactions between hair-inductive dermal cells and epidermal cells are essential for de novo genesis of hair follicles. Recent studies have shown that outer root sheath (ORS) follicular keratinocytes can be expanded in vitro, but the cultured cells often lose receptivity to hair-inducing dermal signals. In this study, we first investigated whether the hair-inductive activity (trichogenicity) of cultured human ORS follicular keratinocytes was correlated with the cultivation period. ORS follicular keratinocytes from the scalp were cultured for 3, 4, 5, or 6 weeks and were then implanted into nude mice along with freshly isolated neonatal mouse dermal cells. We observed that the trichogenicity of the implanted ORS cells was inversely correlated with their cultivation period. These initial findings prompted us to investigate the differentially expressed genes between the short-term (20 days) and long-term (42 days) cultured ORS cells, trichogenic and non-trichogenic, respectively, by microarray analysis. We found that forkhead box protein A2 (FOXA2) was the most up-regulated transcription factor in the trichogenic ORS cells. Thus, we investigated whether the trichogenicity of the cells was affected by FOXA2 expression. We found a significant decrease in the number of induced hair follicles when the ORS cells were transfected with a FOXA2 small interfering RNA versus control small interfering RNA. Taken together, our data strongly suggest that FOXA2 significantly influences the trichogenicity of human ORS cells.
Keywords: forkhead box protein A2; hair induction; outer root sheath; transcription factor; trichogenicity.
Copyright © 2020 Bak, Park, Oh, Kim, Kim and Sung.
Figures


References
-
- Higgins C. A., Chen J. C., Cerise J. E., Jahoda C. A., Christiano A. M. (2013). Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. U.S.A. 110 19679–19688. 10.1073/pnas.1309970110 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

