Developmental acclimation of the thylakoid proteome to light intensity in Arabidopsis
- PMID: 33118270
- PMCID: PMC7898487
- DOI: 10.1111/tpj.15053
Developmental acclimation of the thylakoid proteome to light intensity in Arabidopsis
Abstract
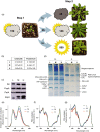
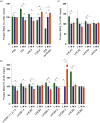
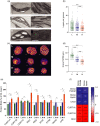
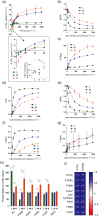
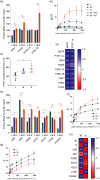
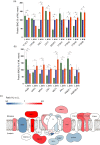
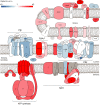
Photosynthetic acclimation, the ability to adjust the composition of the thylakoid membrane to optimise the efficiency of electron transfer to the prevailing light conditions, is crucial to plant fitness in the field. While much is known about photosynthetic acclimation in Arabidopsis, to date there has been no study that combines both quantitative label-free proteomics and photosynthetic analysis by gas exchange, chlorophyll fluorescence and P700 absorption spectroscopy. Using these methods we investigated how the levels of 402 thylakoid proteins, including many regulatory proteins not previously quantified, varied upon long-term (weeks) acclimation of Arabidopsis to low (LL), moderate (ML) and high (HL) growth light intensity and correlated these with key photosynthetic parameters. We show that changes in the relative abundance of cytb6 f, ATP synthase, FNR2, TIC62 and PGR6 positively correlate with changes in estimated PSII electron transfer rate and CO2 assimilation. Improved photosynthetic capacity in HL grown plants is paralleled by increased cyclic electron transport, which positively correlated with NDH, PGRL1, FNR1, FNR2 and TIC62, although not PGR5 abundance. The photoprotective acclimation strategy was also contrasting, with LL plants favouring slowly reversible non-photochemical quenching (qI), which positively correlated with LCNP, while HL plants favoured rapidly reversible quenching (qE), which positively correlated with PSBS. The long-term adjustment of thylakoid membrane grana diameter positively correlated with LHCII levels, while grana stacking negatively correlated with CURT1 and RIQ protein abundance. The data provide insights into how Arabidopsis tunes photosynthetic electron transfer and its regulation during developmental acclimation to light intensity.
Keywords: Acclimation; electron transfer; light harvesting; proteomics; thylakoid.
© 2020 Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Adams, W.W. , Watson, A.M. , Mueh, K.E. , Amiard, V. , Turgeon, R. , Ebbert, V. , Logan, B.A. , Combs, A.F. and Demmig‐Adams, B. (2007) Photosynthetic acclimation in the context of structural constraints to carbon export from leaves. Photosynth. Res. 94, 455–466. - PubMed
-
- Albanese, P. , Manfredi, M. , Meneghesso, A. , Marengo, E. , Saracco, G. , Barber, J. , Morosinotto, T. and Pagliano, C. (2016) Dynamic reorganization of photosystem II supercomplexes in response to variations in light intensities. Biochim. Biophys. Acta – Bioenerg. 1857, 1651–1660. - PubMed
-
- Albanese, P. , Manfredi, M. , Re, A. , Marengo, E. , Saracco, G. and Pagliano, C. (2018) Thylakoid proteome modulation in pea plants grown at different irradiances: quantitative proteomic profiling in a non‐model organism aided by transcriptomic data integration. Plant J. 96, 786–800. - PubMed
-
- Albertsson, P.A. , Andreasson, E. , Stefansson, H. and Wollenberger, L. (1994) Fractionation of thylakoid membrane. Methods Enzymol. 228, 469–482.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

