Glycaemic profiles of diverse patients with type 2 diabetes using basal insulin: MOBILE study baseline data
- PMID: 33118309
- PMCID: PMC7839741
- DOI: 10.1111/dom.14238
Glycaemic profiles of diverse patients with type 2 diabetes using basal insulin: MOBILE study baseline data
Abstract
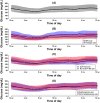
Basal insulin is often prescribed to patients with suboptimally controlled type 2 diabetes (T2D); however, its therapeutic efficacy is inadequate in many. During the MOBILE study's baseline phase, we evaluated 173 participants' continuous glucose monitoring (CGM) data (mean ± SD age 57 ± 9 years; 50% female; HbA1c 9.1% [range 7.1%-11.6%]; 40% using sulphonylureas; 19% using NPH; reported self-monitored blood glucose [SMBG] frequency median 1.0 checks/day) who were using basal, but not prandial insulin. Blinded CGM data were recorded for 10 days prior to randomization. The mean glucose value was 208 ± 47 mg/dL and it was lowest in the early morning. Mean time in the 70-180 mg/dL range was 9.6 ± 6.1 hours/day (40% ± 25%). Hyperglycaemia was extensive with medians of 14.7 (61%) and 5.0 (20.9%) hours/day with glucose greater than 180 and 250 mg/dL, respectively. Hypoglycaemia was infrequent (median [IQR] 0 [0, 4.3] minutes/day [0.0% {0.0%, 0.3%}] with glucose less than 70 mg/dL). Blinded CGM highlights the limitations of infrequent SMBG in basal insulin users with T2D and allows characterization of hyperglycaemia and hypoglycaemia in basal insulin users with suboptimal control. The MOBILE study randomized phase will define the benefits of using real-time CGM compared with SMBG in this population.
Keywords: basal insulin; clinical trial; continuous glucose monitoring.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
AP reports personal fees from Abbott Diabetes Care, Boehringer Ingelheim, Eli Lilly and Company, Livongo, MannKind Corporation, Merck, Novo Nordisk, Sanofi, and Pendulum Therapeutics; grant support from Dexcom and vTv Therapeutics; personal fees from Novo Nordisk; and other support from Mellitus Health, Inc., Omada Health, Inc., Stability Health, LLC, Pendulum Therapeutics, and Livongo outside the submitted work. NC, PC, and KJR have received grant support and donated supplies, paid to the Jaeb Center for Health Research, from Abbott Diabetes Care, Beta Bionics, and Dexcom, and grant support, paid to the Jaeb Center for Health Research, from Tandem Diabetes Care. RWB has received grant support and donated supplies, paid to the Jaeb Center for Health Research, from Abbott Diabetes Care, Ascensia Diabetes Care US, Beta Bionics, and Roche Diabetes Care, grant support, donated supplies, and consulting fees, paid to the Jaeb Center for Health Research, from Dexcom, Novo Nordisk, and Tandem Diabetes Care, grant support and consulting fees, paid to the Jaeb Center for Health Research, from Bigfoot Biomedical, and consulting fees, paid to the Jaeb Center for Health Research, from Eli Lilly and Insulet. TWM has received research support from Abbott Diabetes Care, Dexcom, Lilly, Medtronic, Novo Nordisk and Omnipod. His employer, nonprofit IDC/HealthPartners Institute, contracts for his services and he receives no direct personal income from these activities. SB has received, through her institution, research funding from Dexcom. NMN, SEB, and DAP are employees of Dexcom, Inc.
Figures
References
-
- Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425‐1432. - PubMed
-
- Nichols GA, Romo‐LeTourneau V, Vupputuri S, Thomas SM. Delays in anti‐hyperglycaemic therapy initiation and intensification are associated with cardiovascular events, hospitalizations for heart failure and all‐cause mortality. Diabetes Obes Metab. 2019;21(7):1551‐1557. - PubMed
-
- Karl DM, Gill J, Zhou R, Riddle MC. Clinical predictors of risk of hypoglycaemia during addition and titration of insulin glargine for type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(7):622‐628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

