Uncaria tomentosa (cat's claw): a promising herbal medicine against SARS-CoV-2/ACE-2 junction and SARS-CoV-2 spike protein based on molecular modeling
- PMID: 33118480
- PMCID: PMC7657399
- DOI: 10.1080/07391102.2020.1837676
Uncaria tomentosa (cat's claw): a promising herbal medicine against SARS-CoV-2/ACE-2 junction and SARS-CoV-2 spike protein based on molecular modeling
Abstract
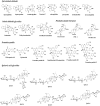
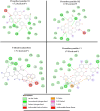
COVID-19 is a novel severe acute respiratory syndrome coronavirus. Currently, there is no effective treatment and vaccines seem to be the solution in the future. Virtual screening of potential drugs against the S protein of severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) has provided small molecular compounds with a high binding affinity. Unfortunately, most of these drugs do not attach with the binding interface of the receptor-binding domain (RBD)-angiotensin-converting enzyme-2 (ACE-2) complex in host cells. Molecular modeling was carried out to evaluate the potential antiviral properties of the components of the medicinal herb Uncaria tomentosa (cat's claw) focusing on the binding interface of the RBD-ACE-2 and the viral spike protein. The in silico approach starts with protein-ligand docking of 26 Cat's claw key components followed by molecular dynamics simulations and re-docked calculations. Finally, we carried out drug-likeness calculations for the most qualified cat's claw components. The structural bioinformatics approaches led to the identification of several bioactive compounds of U. tomentosa with potential therapeutic effect by dual strong interaction with interface of the RBD-ACE-2 and the ACE-2 binding site on SARS-CoV-2 RBD viral spike. In addition, in silico drug-likeness indices for these components were calculated and showed good predicted therapeutic profiles of these phytochemicals found in U. tomentosa (cat's claw). Our findings suggest the potential effectiveness of cat's claw as complementary and/or alternative medicine for COVID-19 treatment.Communicated by Ramaswamy H. Sarma.
Keywords: ACE-2; COVID-19; SARS-CoV-2; Uncaria tomentosa; cat’s claw; molecular modeling; viral spike protein.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



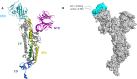



References
-
- Abraham, M. J., Murtola, T., Schulz, R., Páll, S., Smith, J. C., Hess, B., & Lindah, E. (2015). Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX, 1–2, 19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Akram, M., Tahir, I. M., Shah, S. M. A., Mahmood, Z., Altaf, A., Ahmad, K., Munir, N., Daniyal, M., Nasir, S., & Mehboob, H. (2018). Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytotherapy Research, 32(5), 811–822. 10.1002/ptr.6024 - DOI - PubMed
-
- Aquino, R., De Tommasi, N., De Simone, F., & Pizza, C. (1997). Triterpenes and quinovic acid glycosides from Uncaria tomentosa. Phytochemistry, 45(5), 1035–1040. 10.1016/S0031-9422(96)00716-9 - DOI
-
- Araujo, L. C. C., Feitosa, K. B., Murata, G. M., Furigo, I. C., Teixeira, S. A., Lucena, C. F., Ribeiro, L. M., Muscará, M. N., Costa, S. K. P., Donato, J., Bordin, S., Curi, R., & Carvalho, C. R. O. (2018). Uncaria tomentosa improves insulin sensitivity and inflammation in experimental NAFLD. Scientific Reports, 8(1), 11013. 10.1038/s41598-018-29044-y - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
