Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial
- PMID: 33118927
- PMCID: PMC7927315
- DOI: 10.5664/jcsm.8944
Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial
Abstract
Study objectives: Patients with comorbid insomnia and sleep apnea (COMISA) report increased severity of depression, anxiety, and stress symptoms compared to patients with either insomnia or sleep apnea alone. Although cognitive behavioral therapy for insomnia (CBTi) is an effective treatment for COMISA, previous research suggests a reduced response to CBTi by patients with insomnia and depression, anxiety, and stress symptoms. Therefore, we used randomized controlled trial data to investigate the impact of depression, anxiety, and stress symptoms before treatment on changes in insomnia after CBTi vs control in patients with COMISA.
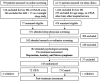
Methods: 145 patients with COMISA (insomnia as defined by the International Classification of Sleep Disorders, third edition and apnea-hypopnea index ≥ 15 events/h) were randomized to CBTi (n = 72) or no-treatment control (n = 73). One-week sleep diaries and standardized questionnaire measures of insomnia, sleepiness, fatigue, depression, anxiety, and stress were completed pretreatment and posttreatment. Mixed models were used to examine interactions between depression, anxiety, and stress symptoms before treatment, intervention-group (CBTi, control), and time (pretreatment, posttreatment) on insomnia symptoms.
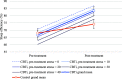
Results: Approximately half of this COMISA sample reported at least mild symptoms of depression (57%), anxiety (53%), and stress (48%) before treatment. Patients reporting greater depression, anxiety, and stress symptoms before treatment also reported increased severity of insomnia, daytime fatigue, and sleepiness. Improvements in questionnaire and diary-measured insomnia symptoms improved during CBTi and were not moderated by severity of depression, anxiety, or stress symptoms before treatment (all interaction P ≥ .11).
Conclusions: We found no evidence that symptoms of depression, anxiety, or stress impair the effectiveness of CBTi in improving insomnia symptoms in patients with COMISA. Patients with COMISA and comorbid symptoms of depression, anxiety, and stress should be referred for CBTi to treat insomnia and improve subsequent management of their obstructive sleep apnea.
Clinical trial registration: Registry: Australian New Zealand Clinical Trials Registry; Name: Treating comorbid insomnia with obstructive sleep apnea (COMISA) study: A new treatment strategy for patients with combined insomnia and sleep apnea; URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365184; Identifier: ACTRN12613001178730.
Keywords: COMISA; anxiety; cognitive behavioral therapy; continuous positive airway pressure therapy; depression; insomnia; obstructive sleep apnea; stress.
© 2021 American Academy of Sleep Medicine.
Conflict of interest statement
All authors have seen and approved this manuscript. Work for this study was performed at the Adelaide Institute for Sleep Health, Flinders University, and The Prince Charles Hospital, Queensland. This study was funded by a National Health and Medical Research Council Grant (1049591; Treating insomnia co-morbid with obstructive sleep apnoea: A randomized controlled clinical effectiveness trial). D.M. reports research funding support from Philips Respironics. P.C. received salary support via an Australian Research Council Future Fellowship (FT120100510) and reports research funding support from Philips Respironics via the CRC for Alertness, Safety and Productivity. D.P. no financial interests to throughout trial period, but began working for Philips Respironics (Melbourne, Australia) following completion of trial data collection. L.L. has received funding support from Re-timer (Re-timer Pty Ltd, Adelaide, Australia). D.M. Research equipment donations from ResMed and Air Liquide. P.G.C. equipment support from Philips Respironics and Air Liquide. C.L.C.C. reports previous equipment support from Philips Respironics and ResMed (ApneaLink devices and CPAP equipment). None of the other authors report conflicts of interest.
Figures


Comment in
-
Impact of CBTi in COMISA: Could it mean a "masking effect" of the circadian time machinery on psychosocial stress factors?J Clin Sleep Med. 2021 Sep 1;17(9):1957-1958. doi: 10.5664/jcsm.9364. J Clin Sleep Med. 2021. PMID: 33960294 Free PMC article. No abstract available.
-
Circadian factors in comorbid insomnia and sleep apnea.J Clin Sleep Med. 2021 Sep 1;17(9):1959-1960. doi: 10.5664/jcsm.9408. Epub 2021 May 12. J Clin Sleep Med. 2021. PMID: 34743791 Free PMC article. No abstract available.
Similar articles
-
The effect of cognitive and behavioral therapy for insomnia on week-to-week changes in sleepiness and sleep parameters in patients with comorbid insomnia and sleep apnea: a randomized controlled trial.Sleep. 2020 Jul 13;43(7):zsaa002. doi: 10.1093/sleep/zsaa002. Sleep. 2020. PMID: 31927569 Clinical Trial.
-
Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial.Sleep. 2019 Dec 24;42(12):zsz178. doi: 10.1093/sleep/zsz178. Sleep. 2019. PMID: 31403168 Clinical Trial.
-
The Effect of Digital Cognitive Behavioural Therapy for Insomnia in People With Co-Morbid Insomnia and Sleep Apnoea (COMISA): A Pilot Randomised Controlled Trial.J Sleep Res. 2025 Jun 25:e70114. doi: 10.1111/jsr.70114. Online ahead of print. J Sleep Res. 2025. PMID: 40557787
-
Comorbid Insomnia and Sleep Apnea: Assessment and Management Approaches.Sleep Med Clin. 2022 Dec;17(4):597-617. doi: 10.1016/j.jsmc.2022.07.006. Epub 2022 Oct 9. Sleep Med Clin. 2022. PMID: 36333079 Review.
-
Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management.Int J Environ Res Public Health. 2021 Sep 1;18(17):9248. doi: 10.3390/ijerph18179248. Int J Environ Res Public Health. 2021. PMID: 34501836 Free PMC article. Review.
Cited by
-
OSA Wellness Scale (OWS): A New Health-Related Quality of Life Test in Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Device.Int J Dent. 2022 Sep 21;2022:4629341. doi: 10.1155/2022/4629341. eCollection 2022. Int J Dent. 2022. PMID: 36187733 Free PMC article.
-
Baseline symptoms of depression and anxiety negatively impact the effectiveness of CBTi in treating acute insomnia among young adults.Gen Psychiatr. 2023 May 22;36(3):e101013. doi: 10.1136/gpsych-2023-101013. eCollection 2023. Gen Psychiatr. 2023. PMID: 37265474 Free PMC article.
-
Effects of combined morbid insomnia and sleep apnea on long-term cardiovascular risk and all-cause mortality in elderly patients: a prospective cohort study.BMC Geriatr. 2024 Jul 21;24(1):622. doi: 10.1186/s12877-024-05147-2. BMC Geriatr. 2024. PMID: 39034410 Free PMC article.
-
Circadian factors in comorbid insomnia and sleep apnea.J Clin Sleep Med. 2021 Sep 1;17(9):1959-1960. doi: 10.5664/jcsm.9408. Epub 2021 May 12. J Clin Sleep Med. 2021. PMID: 34743791 Free PMC article. No abstract available.
-
Sleep Bruxism and Sleep Structure in Comorbid Insomnia and Obstructive Sleep Apnea (COMISA) Syndrome: A Polysomnographic Study.J Clin Med. 2024 May 28;13(11):3154. doi: 10.3390/jcm13113154. J Clin Med. 2024. PMID: 38892864 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

