Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma: A Randomized Clinical Trial
- PMID: 33119048
- PMCID: PMC7596685
- DOI: 10.1001/jamaoncol.2020.3161
Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma: A Randomized Clinical Trial
Abstract
Importance: New treatments are needed to improve the prognosis of patients with recurrent high-grade glioma.
Objective: To compare overall survival for patients receiving tumor resection followed by vocimagene amiretrorepvec (Toca 511) with flucytosine (Toca FC) vs standard of care (SOC).
Design, setting, and participants: A randomized, open-label phase 2/3 trial (TOCA 5) in 58 centers in the US, Canada, Israel, and South Korea, comparing posttumor resection treatment with Toca 511 followed by Toca FC vs a defined single choice of approved (SOC) therapies was conducted from November 30, 2015, to December 20, 2019. Patients received tumor resection for first or second recurrence of glioblastoma or anaplastic astrocytoma.
Interventions: Patients were randomized 1:1 to receive Toca 511/FC (n = 201) or SOC control (n = 202). For the Toca 511/FC group, patients received Toca 511 injected into the resection cavity wall at the time of surgery, followed by cycles of oral Toca FC 6 weeks after surgery. For the SOC control group, patients received investigators' choice of single therapy: lomustine, temozolomide, or bevacizumab.
Main outcomes and measures: The primary outcome was overall survival (OS) in time from randomization date to death due to any cause. Secondary outcomes reported in this study included safety, durable response rate (DRR), duration of DRR, durable clinical benefit rate, OS and DRR by IDH1 variant status, and 12-month OS.
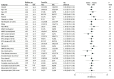
Results: All 403 randomized patients (median [SD] age: 56 [11.46] years; 62.5% [252] men) were included in the efficacy analysis, and 400 patients were included in the safety analysis (3 patients on the SOC group did not receive resection). Final analysis included 271 deaths (141 deaths in the Toca 511/FC group and 130 deaths in the SOC control group). The median follow-up was 22.8 months. The median OS was 11.10 months for the Toca 511/FC group and 12.22 months for the control group (hazard ratio, 1.06; 95% CI 0.83, 1.35; P = .62). The secondary end points did not demonstrate statistically significant differences. The rates of adverse events were similar in the Toca 511/FC group and the SOC control group.
Conclusions and relevance: Among patients who underwent tumor resection for first or second recurrence of glioblastoma or anaplastic astrocytoma, administration of Toca 511 and Toca FC, compared with SOC, did not improve overall survival or other efficacy end points.
Trial registration: ClinicalTrials.gov Identifier: NCT02414165.
Conflict of interest statement
Figures


References
-
- Clarke JL, Ennis MM, Yung WK, et al. ; North American Brain Tumor Consortium . Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro-oncol. 2011;13(10):1118-1124. doi:10.1093/neuonc/nor110 - DOI - PMC - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups . National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi:10.1056/NEJMoa043330 - DOI - PubMed

