Transcriptome and proteome mapping in the sheep atria reveal molecular featurets of atrial fibrillation progression
- PMID: 33119050
- PMCID: PMC8208739
- DOI: 10.1093/cvr/cvaa307
Transcriptome and proteome mapping in the sheep atria reveal molecular featurets of atrial fibrillation progression
Erratum in
-
Erratum to: Transcriptome and proteome mapping in the sheep atria reveal molecular features of atrial fibrillation progression.Cardiovasc Res. 2021 Sep 28;117(11):2353. doi: 10.1093/cvr/cvab216. Cardiovasc Res. 2021. PMID: 34254112 Free PMC article. No abstract available.
Abstract
Aims: Atrial fibrillation (AF) is a progressive cardiac arrhythmia that increases the risk of hospitalization and adverse cardiovascular events. There is a clear demand for more inclusive and large-scale approaches to understand the molecular drivers responsible for AF, as well as the fundamental mechanisms governing the transition from paroxysmal to persistent and permanent forms. In this study, we aimed to create a molecular map of AF and find the distinct molecular programmes underlying cell type-specific atrial remodelling and AF progression.
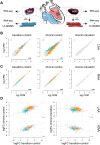
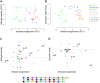
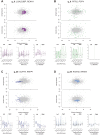
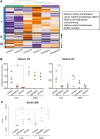
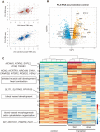
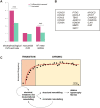
Methods and results: We used a sheep model of long-standing, tachypacing-induced AF, sampled right and left atrial tissue, and isolated cardiomyocytes (CMs) from control, intermediate (transition), and late time points during AF progression, and performed transcriptomic and proteome profiling. We have merged all these layers of information into a meaningful three-component space in which we explored the genes and proteins detected and their common patterns of expression. Our data-driven analysis points at extracellular matrix remodelling, inflammation, ion channel, myofibril structure, mitochondrial complexes, chromatin remodelling, and genes related to neural function, as well as critical regulators of cell proliferation as hallmarks of AF progression. Most important, we prove that these changes occur at early transitional stages of the disease, but not at later stages, and that the left atrium undergoes significantly more profound changes than the right atrium in its expression programme. The pattern of dynamic changes in gene and protein expression replicate the electrical and structural remodelling demonstrated previously in the sheep and in humans, and uncover novel mechanisms potentially relevant for disease treatment.
Conclusions: Transcriptomic and proteomic analysis of AF progression in a large animal model shows that significant changes occur at early stages, and that among others involve previously undescribed increase in mitochondria, changes to the chromatin of atrial CMs, and genes related to neural function and cell proliferation.
Keywords: Atrial fibrillation; Chromatin; Mitochondria; Proteomics; RNA-seq.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
Revealing the molecular history of the transition from paroxysmal to permanent atrial fibrillation.Cardiovasc Res. 2021 Jun 16;117(7):1612-1613. doi: 10.1093/cvr/cvab106. Cardiovasc Res. 2021. PMID: 33878181 No abstract available.
References
-
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. - PMC - PubMed
-
- Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O’Connell RP, Musa H, Guerrero-Serna G, Avula UMR, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–1482. - PMC - PubMed
-
- de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–731. - PubMed
-
- Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 2010;12:160–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

