Safety and efficacy of double vs. triple antithrombotic therapy in patients with atrial fibrillation with or without acute coronary syndrome undergoing percutaneous coronary intervention: a collaborative meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials
- PMID: 33119069
- PMCID: PMC8117456
- DOI: 10.1093/ehjcvp/pvaa116
Safety and efficacy of double vs. triple antithrombotic therapy in patients with atrial fibrillation with or without acute coronary syndrome undergoing percutaneous coronary intervention: a collaborative meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials
Abstract
Aims: Safety and efficacy of antithrombotic regimens in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) may differ based on clinical presentation. We sought to compare double vs. triple antithrombotic therapy (DAT vs. TAT) in AF patients with or without acute coronary syndrome (ACS) undergoing PCI.
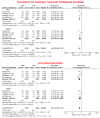
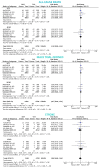
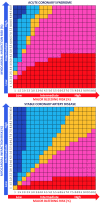
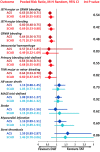
Methods and results: A systematic review and meta-analysis was performed using PubMed to search for non-vitamin K antagonist oral anticoagulant (NOAC)-based randomized clinical trials. Data on subgroups of ACS or elective PCI were obtained by published reports or trial investigators. A total of 10 193 patients from four NOAC trials were analysed, of whom 5675 presenting with ACS (DAT = 3063 vs. TAT = 2612) and 4518 with stable coronary artery disease (SCAD; DAT = 2421 vs. TAT = 2097). The primary safety endpoint of ISTH major bleeding or clinically relevant non-major bleeding was reduced with DAT compared with TAT in both ACS (12.2% vs. 19.4%; RR 0.63, 95% CI 0.56-0.71; P < 0.0001; I2 = 0%) and SCAD (14.6% vs. 22.0%; RR 0.68, 95% CI 0.55-0.85; P = 0.0008; I2 = 66%), without interaction (P-int = 0.54). Findings were consistent for secondary bleeding endpoints, including intra-cranial haemorrhage. In both subgroups, there was no difference between DAT and TAT for all-cause death, major adverse cardiovascular events, or stroke. Myocardial infarction and stent thrombosis were numerically higher with DAT vs. TAT consistently in ACS and SCAD (P-int = 0.60 and 0.86, respectively). Findings were confirmed by multiple sensitivity analyses, including a separate analysis on dabigatran regimens and a restriction to PCI population.
Conclusions: DAT, compared with TAT, is associated with lower bleeding risks, including intra-cranial haemorrhage, and a small non-significant excess of cardiac ischaemic events in both patients with or without ACS.
Keywords: Acute coronary syndrome (ACS); Atrial fibrillation (AF); Double therapy (DAT); Non-vitamin K antagonist oral anticoagulant (NOAC); Percutaneous coronary intervention (PCI); Triple therapy (TAT).
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Comment in
-
Double vs. triple antithrombotic therapy in atrial fibrillation patients undergoing percutaneous coronary intervention: does clinical presentation matter?Eur Heart J Cardiovasc Pharmacother. 2021 Apr 9;7(FI1):f61-f62. doi: 10.1093/ehjcvp/pvaa121. Eur Heart J Cardiovasc Pharmacother. 2021. PMID: 33247917 No abstract available.
-
The multiplication of loaves and fishes approach: a critic to double anti-thrombotics or to double number of ischaemic events?Eur Heart J Cardiovasc Pharmacother. 2021 May 23;7(3):e29-e30. doi: 10.1093/ehjcvp/pvaa141. Eur Heart J Cardiovasc Pharmacother. 2021. PMID: 33340322 Free PMC article. No abstract available.
-
Double or triple antithrombotic therapy for patients with atrial fibrillation undergoing percutaneous coronary intervention: not a matter of faith.Eur Heart J Cardiovasc Pharmacother. 2021 May 23;7(3):e16-e17. doi: 10.1093/ehjcvp/pvaa139. Eur Heart J Cardiovasc Pharmacother. 2021. PMID: 33340325 No abstract available.
Similar articles
-
Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials.Eur Heart J. 2019 Dec 7;40(46):3757-3767. doi: 10.1093/eurheartj/ehz732. Eur Heart J. 2019. PMID: 31651946
-
Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials.Europace. 2020 Jan 1;22(1):33-46. doi: 10.1093/europace/euz259. Europace. 2020. PMID: 31603196
-
Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients.Europace. 2020 Apr 1;22(4):538-546. doi: 10.1093/europace/euz345. Europace. 2020. PMID: 31942971
-
Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials.Int J Cardiol. 2020 Mar 1;302:95-102. doi: 10.1016/j.ijcard.2019.12.054. Epub 2019 Dec 28. Int J Cardiol. 2020. PMID: 31924397
-
Antithrombotic strategies in patients needing oral anticoagulation undergoing percutaneous coronary intervention: A network meta-analysis.Catheter Cardiovasc Interv. 2021 Mar;97(4):581-588. doi: 10.1002/ccd.29192. Epub 2020 Aug 13. Catheter Cardiovasc Interv. 2021. PMID: 32790145
Cited by
-
Apixaban versus PhenpRocoumon: Oral AntiCoagulation plus antiplatelet tHerapy in patients with Acute Coronary Syndrome and Atrial Fibrillation (APPROACH-ACS-AF): Rationale and design of the prospective randomized parallel-group, open-label, blinded-endpoint, superiority, multicenter-trial of a triple therapy versus a dual therapy in patients with Atrial Fibrillation and Acute Coronary Syndrome undergoing coronary stenting.Int J Cardiol Heart Vasc. 2021 Jul 1;35:100810. doi: 10.1016/j.ijcha.2021.100810. eCollection 2021 Aug. Int J Cardiol Heart Vasc. 2021. PMID: 34258380 Free PMC article.
-
Opportunità cliniche e impatto sul sistema sanitario di un trattamento ottimale del paziente post-sindrome coronarica acuta.Glob Reg Health Technol Assess. 2022 May 24;9(Suppl 1):17-26. doi: 10.33393/grhta.2022.2391. eCollection 2022 Jan-Dec. Glob Reg Health Technol Assess. 2022. PMID: 36628067 Free PMC article. Review. Italian.
-
Dual versus triple antithrombotic therapy after percutaneous coronary intervention: the prospective multicentre WOEST 2 Study.EuroIntervention. 2022 Jul 22;18(4):e303-e313. doi: 10.4244/EIJ-D-21-00703. EuroIntervention. 2022. PMID: 35370126 Free PMC article.
-
The multiplication of loaves and fishes approach: a critic to double anti-thrombotics or to double number of ischaemic events?Eur Heart J Cardiovasc Pharmacother. 2021 May 23;7(3):e29-e30. doi: 10.1093/ehjcvp/pvaa141. Eur Heart J Cardiovasc Pharmacother. 2021. PMID: 33340322 Free PMC article. No abstract available.
-
Procedural and Antithrombotic Therapy Optimization in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Narrative Review.J Cardiovasc Dev Dis. 2025 Apr 8;12(4):142. doi: 10.3390/jcdd12040142. J Cardiovasc Dev Dis. 2025. PMID: 40278201 Free PMC article. Review.
References
-
- Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP.. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective-2018 update. Circulation 2018;138:527–536. - PubMed
-
- Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, Vranckx P, Lopes RD, Montalescot G, Cannon CP, Ten Berg J, Gersh BJ, Bhatt DL, Angiolillo DJ.. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:83–99. - PubMed
-
- Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann F-J, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. - PubMed
-
- Gargiulo G, Windecker S, Vranckx P, Gibson CM, Mehran R, Valgimigli M.. A critical appraisal of aspirin in secondary prevention: is less more? Circulation 2016;134:1881–1906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

