Mechanism of fibrosis and stricture formation in Crohn's disease
- PMID: 33119150
- PMCID: PMC7757243
- DOI: 10.1111/sji.12990
Mechanism of fibrosis and stricture formation in Crohn's disease
Abstract
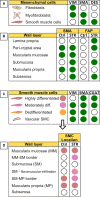
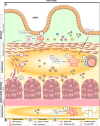
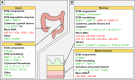
Crohn's disease (CD) is a chronic inflammatory disease of the gastrointestinal tract that leads to substantial suffering for millions of patients. In some patients, the chronic inflammation leads to remodelling of the extracellular matrix and fibrosis. Fibrosis, in combination with expansion of smooth muscle layers, leaves the bowel segment narrowed and stiff resulting in strictures, which often require urgent medical intervention. Although stricture development is associated with inflammation in the affected segment, anti-inflammatory therapies fall far short of treating strictures. At best, current therapies might allow some patients to avoid surgery in a shorter perspective and no anti-fibrotic therapy is yet available. This likely relates to our poor understanding of the mechanism underlying stricture development. Chronic inflammation is a prerequisite, but progression to strictures involves changes in fibroblasts, myofibroblasts and smooth muscle cells in a poorly understood interplay with immune cells and environmental cues. Much of the experimental evidence available is from animal models, cell lines or non-strictured patient tissue. Accordingly, these limitations create the basis for many previously published reviews covering the topic. Although this information has contributed to the understanding of fibrotic mechanisms in general, in the end, data must be validated in strictured tissue from patients. As stricture formation is a serious complication of CD, we endeavoured to summarize findings exclusively performed using strictured tissue from patients. Here, we give an update of the mechanism driving this serious complication in patients, and how the strictured tissue differs from adjacent unaffected tissue and controls.
Keywords: Crohn's disease; fibrosis; strictures.
© 2020 The Authors. Scandinavian Journal of Immunology published by John Wiley & Sons Ltd on behalf of The Scandinavian Foundation for Immunology.
Conflict of interest statement
Both authors declare no conflict of interest.
Figures


References
-
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46‐54.e42; quiz e30. - PubMed
-
- Torres J, Mehandru S, Colombel J‐F, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741‐1755. - PubMed
-
- Cosnes J, Cattan S, Blain A, et al. Long‐term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8(4):244‐250. - PubMed
-
- Rieder F, Latella G, Magro F, et al. European Crohn's and colitis organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn's disease. J Crohns Colitis. 2016;10(8):873‐885. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

