Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis
- PMID: 33119402
- PMCID: PMC7781141
- DOI: 10.1164/rccm.202006-2405OC
Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis
Abstract
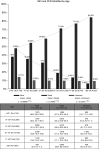
Rationale: Initial reports of case fatality rates (CFRs) among adults with coronavirus disease (COVID-19) receiving invasive mechanical ventilation (IMV) are highly variable.Objectives: To examine the CFR of patients with COVID-19 receiving IMV.Methods: Two authors independently searched PubMed, Embase, medRxiv, bioRxiv, the COVID-19 living systematic review, and national registry databases. The primary outcome was the "reported CFR" for patients with confirmed COVID-19 requiring IMV. "Definitive hospital CFR" for patients with outcomes at hospital discharge was also investigated. Finally, CFR was analyzed by patient age, geographic region, and study quality on the basis of the Newcastle-Ottawa Scale.Measurements and Results: Sixty-nine studies were included, describing 57,420 adult patients with COVID-19 who received IMV. Overall reported CFR was estimated as 45% (95% confidence interval [CI], 39-52%). Fifty-four of 69 studies stated whether hospital outcomes were available but provided a definitive hospital outcome on only 13,120 (22.8%) of the total IMV patient population. Among studies in which age-stratified CFR was available, pooled CFR estimates ranged from 47.9% (95% CI, 46.4-49.4%) in younger patients (age ≤40 yr) to 84.4% (95% CI, 83.3-85.4%) in older patients (age >80 yr). CFR was also higher in early COVID-19 epicenters. Overall heterogeneity is high (I2 >90%), with nonsignificant Egger's regression test suggesting no publication bias.Conclusions: Almost half of patients with COVID-19 receiving IMV died based on the reported CFR, but variable CFR reporting methods resulted in a wide range of CFRs between studies. The reported CFR was higher in older patients and in early pandemic epicenters, which may be influenced by limited ICU resources. Reporting of definitive outcomes on all patients would facilitate comparisons between studies.Systematic review registered with PROSPERO (CRD42020186997).
Keywords: COVID-19; SARS-CoV-2; case fatality rate; invasive mechanical ventilation; mortality.
Figures


Comment in
-
Estimating the Case Fatality Risk of COVID-19 among Mechanically Ventilated Patients.Am J Respir Crit Care Med. 2021 Jan 1;203(1):3-4. doi: 10.1164/rccm.202011-4117ED. Am J Respir Crit Care Med. 2021. PMID: 33207122 Free PMC article. No abstract available.
References
-
- World Health Organization. Geneva, Switzerland: World Health Organization; 2020. Coronavirus disease (COVID-19) situation reports. [accessed 2020 Jul 30]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. [online ahead of print] 17 Jul 2020; DOI: 10.1056/NEJMoa2021436.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

