B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study
- PMID: 33119478
- PMCID: PMC7768336
- DOI: 10.1200/JCO.20.01974
B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study
Abstract
Purpose: Desmoplastic small round cell tumor (DSRCT), a rare sarcoma of adolescents/young adults primarily involving the peritoneum, has a long-term survival of < 20% despite aggressive multimodality treatment. B7H3 is expressed on DSRCT cell surface, providing a target for antibody-based immunotherapy.
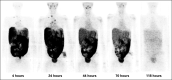
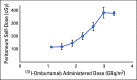
Patients and methods: In this phase I study, we evaluated the safety, pharmacokinetics, and biodistribution of intraperitoneal (IP) radioimmunotherapy (RIT) with the anti-B7H3 murine monoclonal antibody 131I-omburtamab in patients with DSRCT or other B7H3-expressing tumors involving the peritoneum. After thyroid blockade, patients received 131I-omburtamab as a single IP injection at escalated activities from 1.11 to 3.33/GBq/m2. A prior tracer dose of IP 74 MBq124I-omburtamab was used for radioimmuno-positron emission tomography imaging. Each injection was followed by IP saline infusion.
Results: Fifty-two patients (48, three, and one with DSRCT, peritoneal rhabdomyosarcoma, and Ewing sarcoma, respectively) received IP 131I-omburtamab administered on an outpatient basis. Maximum tolerated dose was not reached; there were no dose-limiting toxicities. Major related adverse events were transient: grade 4 neutropenia (n = 2 patients) and thrombocytopenia (n = 1), and grade 1 (10%) and grade 2 (52%) pain lasting < 2 hours related to saline infusion. Hypothyroidism was not observed, and antidrug antibody was elicited in 5%. Mean (± SD) projected peritoneal residence time was 22.4 ± 7.9 hours. Mean projected absorbed doses for 131I-omburtamab based on 124I-omburtamab dosimetry to normal organs were low and well within tolerable limits. More than 80% 131I remained protein bound in blood 66 hours after RIT. On the basis of peritoneal dose and feasibility for outpatient administration, the recommended phase II activity was established at 2.96 GBq/m2. Patients with DSRCT receiving standard whole-abdominal radiotherapy after RIT did not experience unexpected toxicity.
Conclusion: IP RIT 131I-omburtamab was well tolerated with minimal toxicities. Radiation exposure to normal organs was low, making combination therapy with other anticancer therapies feasible.
Trial registration: ClinicalTrials.gov NCT01099644.
Figures
References
-
- Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499–513. - PubMed
-
- Sawyer JR, Tryka AF, Lewis JM. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. Am J Surg Pathol. 1992;16:411–416. - PubMed
-
- Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837–2840. - PubMed
-
- Gerald WL, Haber DA. The EWS-WT1 gene fusion in desmoplastic small round cell tumor. Semin Cancer Biol. 2005;15:197–205. - PubMed
-
- Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: Prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14:1526–1531. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials