Dietary Supplement Use in Children and Adolescents Aged ≤19 Years - United States, 2017-2018
- PMID: 33119556
- PMCID: PMC7641005
- DOI: 10.15585/mmwr.mm6943a1
Dietary Supplement Use in Children and Adolescents Aged ≤19 Years - United States, 2017-2018
Abstract
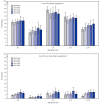
Dietary supplement use is common among children and adolescents. During 2013-2014, approximately one third of children and adolescents (persons aged ≤19 years) in the United States were reported to use a dietary supplement in the past 30 days, and use varied by demographic characteristics (1,2). Dietary supplements can contribute substantially to overall nutrient intake, having the potential to both mitigate nutrient shortfalls as well as to lead to nutrient intake above recommended upper limits (3). However, because nutritional needs should generally be met through food consumption according to the 2015-2020 Dietary Guidelines for Americans, only a few dietary supplements are specifically recommended for use among children and adolescents and only under particular conditions (4). The most recently released data from the National Health and Nutrition Examination Survey (NHANES) during 2017-2018 were used to estimate the prevalence of use among U.S. children and adolescents of any dietary supplement, two or more dietary supplements, and specific dietary supplement product types. Trends were calculated for dietary supplement use from 2009-2010 to 2017-2018. During 2017-2018, 34.0% of children and adolescents used any dietary supplement in the past 30 days, with no significant change since 2009-2010. Use of two or more dietary supplements increased from 4.3% during 2009-2010 to 7.1% during 2017-2018. Multivitamin-mineral products were used by 23.8% of children and adolescents, making these the products most commonly used. Because dietary supplement use is common, surveillance of dietary supplement use, combined with nutrient intake from diet, will remain an important component of monitoring nutritional intake in children and adolescents to inform clinical practice and dietary recommendations.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- US Department of Health and Human Services; US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington, DC; 2015. https://health.gov/dietaryguidelines/2015/guidelines/
-
- Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol 1998;24:193–201.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

