Effect of disease duration in a randomized Phase III trial of rintatolimod, an immune modulator for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
- PMID: 33119613
- PMCID: PMC7595369
- DOI: 10.1371/journal.pone.0240403
Effect of disease duration in a randomized Phase III trial of rintatolimod, an immune modulator for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
Background: Rintatolimod is a selective TLR3 agonist, which has demonstrated clinical activity for ME/CFS in Phase II and Phase III double-blind, placebo-controlled, randomized, multi-site clinical trials.
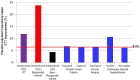
Methods and findings: A hypothesis-based post-hoc analysis of the Intent to Treat (ITT) population diagnosed with ME/CFS from 12 independent clinical sites of a Phase III trial was performed to evaluate the effect of rintatolimod therapy based on disease duration. The clinical activity of rintatolimod was evaluated by exercise treadmill tolerance (ETT) using a modified Bruce protocol. The ITT population (n = 208) was divided into two subsets of symptom duration. Patients with symptom duration of 2-8 years were identified as the Target Subset (n = 75); the remainder (<2 year plus >8 year) were identified as the Non-Target Subset (n = 133). Placebo-adjusted percentage improvements in exercise duration and the vertical rise for the Target Subset (n = 75) were more than twice that of the ITT population. The Non-Target Subset (n = 133) failed to show any clinically significant ETT response to rintatolimod when compared to placebo. Within the Target Subset, 51.2% of rintatolimod-treated patients improved their exercise duration by ≥25% (p = 0.003) despite reduced statistical power from division of the original ITT population into two subsets.
Conclusion/significance: Analysis of ETT from a Phase III trial has identified within the ITT population, a subset of ME/CFS patients with ≥2 fold increased exercise response to rintatolimod. Substantial improvement in physical performance was seen for the majority (51.2%) of these severely debilitated patients who improved exercise duration by ≥25%. This magnitude of exercise improvement was associated with clinically significant enhancements in quality of life. The data indicate that ME/CFS patients have a relatively short disease duration window (<8 years) to expect a significant response to rintatolimod under the dosing conditions utilized in this Phase III clinical trial. These results may have direct relevance to the cognitive impairment and fatigue being experienced by patients clinically recovered from COVID-19 and free of detectable SARS-CoV-2.
Trial registration: ClinicalTrials.gov: NCT00215800.
Conflict of interest statement
We have the following interests: DRS is the Chief Scientific Officer and Medical Director and DLY is the Clinical Project Manager for AIM ImmunoTech with minor equity holdings in the company. WMM is the independent Chairman of the Board of Directors of AIM ImmunoTech with an equity interest, and a member of the Board for Chronix Biomedical. Rintatolimod is a product in development for ME/CFS with a pending patent application. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures


References
-
- Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, et al. Chronic Fatigue Syndrome: A Working Case Definition. Ann Intern Med 1988;108:387–89. - PubMed
-
- Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J Chronic Fatigue Syndrome 2003;11:7–36.
-
- mereserach.org [internet]. Energizing ME Research, c2019 [cited 2019 Feb 25]. ME/CFS in Women and Men; http://www.meresearch.org.uk/news/sex-differences-in-mecfs/
-
- ammes.org [internet]. American Myalgic Encephalomyelitis and Chronic Fatigue Syndrome Society [cited 2019 Feb 25]. How Many People Have ME/CFS; https://ammes.org/how-many-people-have-mecfs/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

