Tethering-facilitated DNA 'opening' and complementary roles of β-hairpin motifs in the Rad4/XPC DNA damage sensor protein
- PMID: 33119737
- PMCID: PMC7708039
- DOI: 10.1093/nar/gkaa909
Tethering-facilitated DNA 'opening' and complementary roles of β-hairpin motifs in the Rad4/XPC DNA damage sensor protein
Abstract
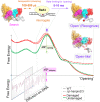
XPC/Rad4 initiates eukaryotic nucleotide excision repair on structurally diverse helix-destabilizing/distorting DNA lesions by selectively 'opening' these sites while rapidly diffusing along undamaged DNA. Previous structural studies showed that Rad4, when tethered to DNA, could also open undamaged DNA, suggesting a 'kinetic gating' mechanism whereby lesion discrimination relied on efficient opening versus diffusion. However, solution studies in support of such a mechanism were lacking and how 'opening' is brought about remained unclear. Here, we present crystal structures and fluorescence-based conformational analyses on tethered complexes, showing that Rad4 can indeed 'open' undamaged DNA in solution and that such 'opening' can largely occur without one or the other of the β-hairpin motifs in the BHD2 or BHD3 domains. Notably, the Rad4-bound 'open' DNA adopts multiple conformations in solution notwithstanding the DNA's original structure or the β-hairpins. Molecular dynamics simulations reveal compensatory roles of the β-hairpins, which may render robustness in dealing with and opening diverse lesions. Our study showcases how fluorescence-based studies can be used to obtain information complementary to ensemble structural studies. The tethering-facilitated DNA 'opening' of undamaged sites and the dynamic nature of 'open' DNA may shed light on how the protein functions within and beyond nucleotide excision repair in cells.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


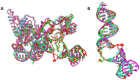


References
-
- Hoogstraten D., Bergink S., Ng J.M., Verbiest V.H., Luijsterburg M.S., Geverts B., Raams A., Dinant C., Hoeijmakers J.H., Vermeulen W. et al. .. Versatile DNA damage detection by the global genome nucleotide excision repair protein XPC. J. Cell Sci. 2008; 121:2850–2859. - PubMed
-
- Sugasawa K., Ng J.M., Masutani C., Iwai S., van der Spek P.J., Eker A.P., Hanaoka F., Bootsma D., Hoeijmakers J.H.. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell. 1998; 2:223–232. - PubMed
-
- Wood R.D. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie. 1999; 81:39–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

