Off-label use of dexmedetomidine in paediatric anaesthesiology: an international survey of 791 (paediatric) anaesthesiologists
- PMID: 33119787
- PMCID: PMC7935836
- DOI: 10.1007/s00228-020-03028-2
Off-label use of dexmedetomidine in paediatric anaesthesiology: an international survey of 791 (paediatric) anaesthesiologists
Abstract
Purpose: The purpose of this international study was to investigate prescribing practices of dexmedetomidine by paediatric anaesthesiologists.
Methods: We performed an online survey on the prescription rate of dexmedetomidine, route of administration and dosage, adverse drug reactions, education on the drug and overall experience. Members of specialist paediatric anaesthesia societies of Europe (ESPA), New Zealand and Australia (SPANZA), Great Britain and Ireland (APAGBI) and the USA (SPA) were consulted. Responses were collected in July and August 2019.
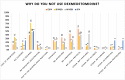
Results: Data from 791 responders (17% of 5171 invitees) were included in the analyses. Dexmedetomidine was prescribed by 70% of the respondents (ESPA 53%; SPANZA 69%; APAGBI 34% and SPA 96%), mostly for procedural sedation (68%), premedication (46%) and/or ICU sedation (46%). Seventy-three percent had access to local or national protocols, although lack of education was the main reason cited by 26% of the respondents not to prescribe dexmedetomidine. The main difference in dexmedetomidine use concerned the age of patients (SPA primarily < 1 year, others primarily > 1 year). The dosage varied widely ranging from 0.2-5 μg kg-1 for nasal premedication, 0.2-8 μg kg-1 for nasal procedural sedation and 0-4 μg kg-1 intravenously as adjuvant for anaesthesia. Only ESPA members (61%) had noted an adverse drug reaction, namely bradycardia.
Conclusion: The majority of anaesthesiologists use dexmedetomidine in paediatrics for premedication, procedural sedation, ICU sedation and anaesthesia, despite the off-label use and sparse evidence. The large intercontinental differences in prescribing dexmedetomidine call for consensus and worldwide education on the optimal use in paediatric practice.
Keywords: Anaesthesia; Dexmedetomidine; Drug prescriptions; Off-label use; Paediatrics; Pharmacology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Szmuk P, Andropoulos D, McGowan F, Brambrink A, Lee C, Lee KJ, McCann ME, Liu Y, Saynhalath R, Bong CL, Anderson BJ, Berde C, De Graaff JC, Disma N, Kurth D, Loepke A, Orser B, Sessler DI, Skowno JJ, von Ungern-Sternberg BS, Vutskits L, Davidson A. An open label pilot study of a dexmedetomidine-remifentanil-caudal anesthetic for infant lower abdominal/lower extremity surgery: the T REX pilot study. Paediatr Anaesth. 2019;29(1):59–67. doi: 10.1111/pan.13544. - DOI - PubMed
-
- Gupta A, Dalvi NP, Tendolkar BA. Comparison between intranasal dexmedetomidine and intranasal midazolam as premedication for brain magnetic resonance imaging in pediatric patients: a prospective randomized double blind trial. J Anaesthesiol Clin Pharmacol. 2017;33(2):236–240. doi: 10.4103/joacp.JOACP_204_16. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous