Switching to Biosimilar SDZ-ADL in Patients with Moderate-to-Severe Active Rheumatoid Arthritis: 48-Week Efficacy, Safety and Immunogenicity Results From the Phase III, Randomized, Double-Blind ADMYRA Study
- PMID: 33119861
- PMCID: PMC7669771
- DOI: 10.1007/s40259-020-00447-6
Switching to Biosimilar SDZ-ADL in Patients with Moderate-to-Severe Active Rheumatoid Arthritis: 48-Week Efficacy, Safety and Immunogenicity Results From the Phase III, Randomized, Double-Blind ADMYRA Study
Abstract
Background: Sandoz adalimumab SDZ-ADL (GP-2017) is an approved adalimumab biosimilar with similar efficacy and comparable safety and immunogenicity to reference adalimumab (ref-ADL) as confirmed by analytical, pharmacokinetic and confirmatory studies. ADMYRA, a phase III double-blind study, was conducted with an aim to generate efficacy, safety and immunogenicity comparability data in patients with moderate-to-severe rheumatoid arthritis (RA) having inadequate response to disease-modifying anti-rheumatic drugs (DMARDs) including methotrexate (MTX). The study also evaluated an aspect of 'switching' reference product to the biosimilar in terms of efficacy, safety and immunogenicity up to Week 48.
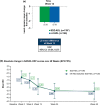
Methods: Eligible patients (N = 353) were randomized 1:1 to receive subcutaneous (sc) SDZ-ADL 40 mg (n = 177) or ref-ADL (n = 176) every other week from Week 0 to Week 24. At Week 24, all patients with at least a moderate response by Disease Activity Score-28 including high-sensitivity C-reactive protein (DAS28-CRP) in the SDZ-ADL group continued SDZ-ADL (n = 159), and in the ref-ADL group were switched to SDZ-ADL (n = 166), treated for up to 46 weeks. The primary endpoint was change in DAS28-CRP from baseline at Week 12. Other efficacy endpoints included proportion of patients with European League Against Rheumatism (EULAR) response, EULAR remission, Boolean remission, safety and immunogenicity.
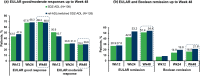
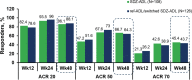
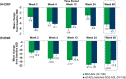
Results: The DAS28-CRP score changes from baseline at Week 12 were similar between SDZ-ADL (- 2.16) and ref-ADL (- 2.18) with a mean difference (95% CI) of 0.02 (- 0.24 to 0.27), which was within the pre-specified equivalence margin of ± 0.6. After switching treatment from ref-ADL to SDZ-ADL, the mean DAS28-CRP change was similar between the SDZ-ADL and 'ref-ADL/switched SDZ-ADL' group (- 3.09 vs - 3.05). The proportion of patients with good/moderate EULAR response was 69.2%/29.0% in the SDZ-ADL group and 68.0%/29.6% in the 'ref-ADL/switched SDZ-ADL' group. The proportion of patients in EULAR remission was 51.4% and 54.4% and in Boolean remission was 16.8% and 21.6% for SDZ-ADL and 'ref-ADL/switched SDZ-ADL' groups, respectively. The secondary endpoints were similar across the treatment groups. The incidence of adverse events (AEs) and injection-site reactions were low and similar between SDZ-ADL and 'ref-ADL/switched SDZ-ADL' groups (AEs 70.6% vs 68.8%, injection-site reactions 4.0% vs 6.3%), and most of these patients experienced AEs of mild or moderate severity. Antidrug antibodies were detected in 24.2% and 25.6% of patients treated with SDZ-ADL and 'ref-ADL/switched SDZ-ADL', respectively, from baseline to Week 48, of which 72.5% in SDZ-ADL and 79.1% in 'ref-ADL/switched SDZ-ADL' groups were neutralizing.
Conclusions: In patients with moderate-to-severe RA who had an inadequate response to DMARDs, SDZ-ADL demonstrated a similar efficacy and a comparable safety and immunogenicity profile to ref-ADL. Efficacy was sustained after switching from ref-ADL to SDZ-ADL with no impact on safety (NCT02744755).
Conflict of interest statement
Piotr Wiland: None declared. Sławomir Jeka: None declared. Eva Dokoupilová: Research grants from AbbVie, Eli Lilly, GSK, Novartis, Pfizer, UCB, Sanofi-Aventis and Hexal AG. Jan Brandt-Jürgens: None declared. Juan Manuel Miranda Limón: None declared. Miguel Cantalejo Moreira: None declared. Raul Veiga Cabello: None declared. Julia Jauch-Lembach: Employee of Sandoz. Anjali Thakur: Employee of Sandoz. Halimuniyazi Haliduola: Employee of Sandoz. Ines Brueckmann: Employee of Sandoz. Norman B. Gaylis: None declared.
Figures

References
-
- EuropeanMedicinesAgency. Biosimilars in the EU: Information guide for healthcare professionals. 2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-informatio.... Accessed 07 May 2019.
-
- EuropeanMedicinesAgency. Guideline on similar biological medicinal products containing monoclonal antibodies—non-clinical and clinical issues 2012. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-si.... Accessed 07 May 2019.
-
- FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

