Effect of Particle Wettability and Particle Concentration on the Enzymatic Dehydration of n-Octanaloxime in Pickering Emulsions
- PMID: 33119950
- PMCID: PMC7839585
- DOI: 10.1002/anie.202013171
Effect of Particle Wettability and Particle Concentration on the Enzymatic Dehydration of n-Octanaloxime in Pickering Emulsions
Abstract
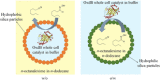
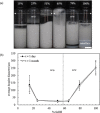
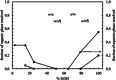
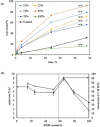
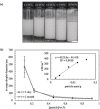
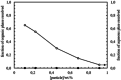
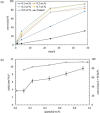
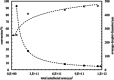
Pickering emulsion systems have emerged as platforms for the synthesis of organic molecules in biphasic biocatalysis. Herein, the catalytic performance was evaluated for biotransformation using whole cells exemplified for the dehydration of n-octanaloxime to n-octanenitrile catalysed by an aldoxime dehydratase (OxdB) overexpressed in E. coli. This study was carried out in Pickering emulsions stabilised solely with silica particles of different hydrophobicity. We correlate, for the first time, the properties of the emulsions with the conversion of the reaction, thus gaining an insight into the impact of the particle wettability and particle concentration. When comparing two emulsions of different type with similar stability and droplet diameter, the oil-in-water (o/w) system displayed a higher conversion than the water-in-oil (w/o) system, despite the conversion in both cases being higher than that in a "classic" two-phase system. Furthermore, an increase in particle concentration prior to emulsification resulted in an increase of the interfacial area and hence a higher conversion.
Keywords: Pickering emulsions; biphasic catalysis; enzyme catalysis; silica particles.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Chen Z., Zhou L., Bing W., Zhang Z., Li Z., Ren J., Qu X., J. Am. Chem. Soc. 2014, 136, 7498–7504. - PubMed
-
- Schmid A., Dordick J. S., Hauer B., Kiener A., Wubbolts M., Witholt B., Nature 2001, 409, 258–268. - PubMed
-
- Carrea G., Riva S., Angew. Chem. Int. Ed. 2000, 39, 2226–2254; - PubMed
- Angew. Chem. 2000, 112, 2312–2341.
-
- Hinzmann A., Glinski S., Worm M., Gröger H., J. Org. Chem. 2019, 84, 4867–4872. - PubMed
-
- Hashimoto T., Maruoka K., Chem. Rev. 2007, 107, 5656–5682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

