Translating an evidence-based clinical pathway into shareable CDS: developing a systematic process using publicly available tools
- PMID: 33120411
- PMCID: PMC7810443
- DOI: 10.1093/jamia/ocaa257
Translating an evidence-based clinical pathway into shareable CDS: developing a systematic process using publicly available tools
Abstract
Objective: To develop a process for translating semi-structured clinical decision support (CDS) into shareable, computer-readable CDS.
Materials and methods: We developed a systematic and transparent process using publicly available tools (eGLIA, GEM Cutter, VSAC, and the CDS Authoring Tool) to translate an evidence-based clinical pathway (CP) into a Clinical Quality Language (CQL)-encoded CDS artifact.
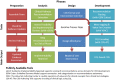
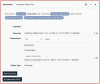
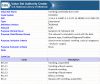
Results: We produced a 4-phase process for translating a CP into a CQL-based CDS artifact. CP content was extracted using GEM into discrete clinical concepts, encoded using standard terminologies into value sets on VSAC, evaluated against workflows using a wireframe, and finally structured as a computer readable CDS artifact using CQL. This process included a quality control step and intermediate products to support transparency and reuse by other CDS developers.
Discussion: Translating a CP into a shareable, computer-readable CDS artifact was accomplished through a systematic process. Our process identified areas of ambiguity and gaps in the CP, which generated improvements in the CP. Collaboration with clinical subject experts and the CP development team was essential for translation. Publicly available tools were sufficient to support most translation steps, but expression of certain complex concepts required manual encoding.
Conclusion: Standardized development of CDS from a CP is feasible using a systematic 4-phase process. CPs represent a potential reservoir for developers of evidence-based CDS. Aspects of CP development simplified portions of the CDS translation process. Publicly available tools can facilitate CDS development; however, enhanced tool features are needed to model complex CDS statements.
Keywords: clinical decision support; clinical quality language; pathways, dissemination, Clostridoides difficile.
© The Author(s) 2020. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Forrest CB, Fiks AG, Bailey LC, et al. Improving adherence to otitis media guidelines with clinical decision support and physician feedback. Pediatrics 2013; 131 (4): e1071–e1081. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

