Lipid metabolic Reprogramming: Role in Melanoma Progression and Therapeutic Perspectives
- PMID: 33121001
- PMCID: PMC7692067
- DOI: 10.3390/cancers12113147
Lipid metabolic Reprogramming: Role in Melanoma Progression and Therapeutic Perspectives
Abstract
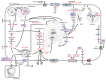
Metabolic reprogramming contributes to the pathogenesis and heterogeneity of melanoma. It is driven both by oncogenic events and the constraints imposed by a nutrient- and oxygen-scarce microenvironment. Among the most prominent metabolic reprogramming features is an increased rate of lipid synthesis. Lipids serve as a source of energy and form the structural foundation of all membranes, but have also emerged as mediators that not only impact classical oncogenic signaling pathways, but also contribute to melanoma progression. Various alterations in fatty acid metabolism have been reported and can contribute to melanoma cell aggressiveness. Elevated expression of the key lipogenic fatty acid synthase is associated with tumor cell invasion and poor prognosis. Fatty acid uptake from the surrounding microenvironment, fatty acid β-oxidation and storage also appear to play an essential role in tumor cell migration. The aim of this review is (i) to focus on the major alterations affecting lipid storage organelles and lipid metabolism. A particular attention has been paid to glycerophospholipids, sphingolipids, sterols and eicosanoids, (ii) to discuss how these metabolic dysregulations contribute to the phenotype plasticity of melanoma cells and/or melanoma aggressiveness, and (iii) to highlight therapeutic approaches targeting lipid metabolism that could be applicable for melanoma treatment.
Keywords: cancer; cholesterol; eicosanoid; fatty acid; glycerophospholipid; lipid droplet; metastasis; obesity; phenotypic switch; pseudo-EMT; sphingolipid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Baenke F., Dhomen N., Gottlieb E., Marais R. Melanoma metabolism. In: Fisher D.E., Bastian B.C., editors. Melanoma. Springer; New York, NY, USA: 2019. - DOI
-
- Fischer G.M., Vashisht Gopal Y.N., McQuade J.L., Peng W., DeBerardinis R.J., Davies M.A. Metabolic strategies of melanoma cells: Mechanisms, interactions with the tumor microenvironment, and therapeutic implications. Pigment Cell Melanoma. Res. 2018;31:11–30. doi: 10.1111/pcmr.12661. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

