Prion-Like Propagation Mechanisms in Tauopathies and Traumatic Brain Injury: Challenges and Prospects
- PMID: 33121065
- PMCID: PMC7692808
- DOI: 10.3390/biom10111487
Prion-Like Propagation Mechanisms in Tauopathies and Traumatic Brain Injury: Challenges and Prospects
Abstract
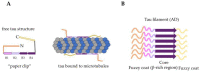
The accumulation of tau protein in the form of filamentous aggregates is a hallmark of many neurodegenerative diseases such as Alzheimer's disease (AD) and chronic traumatic encephalopathy (CTE). These dementias share traumatic brain injury (TBI) as a prominent risk factor. Tau aggregates can transfer between cells and tissues in a "prion-like" manner, where they initiate the templated misfolding of normal tau molecules. This enables the spread of tau pathology to distinct parts of the brain. The evidence that tauopathies spread via prion-like mechanisms is considerable, but work detailing the mechanisms of spread has mostly used in vitro platforms that cannot fully reveal the tissue-level vectors or etiology of progression. We review these issues and then briefly use TBI and CTE as a case study to illustrate aspects of tauopathy that warrant further attention in vivo. These include seizures and sleep/wake disturbances, emphasizing the urgent need for improved animal models. Dissecting these mechanisms of tauopathy progression continues to provide fresh inspiration for the design of diagnostic and therapeutic approaches.
Keywords: clearance; concussion; dementia; prionoid; proteinopathies; seeding; strains; tau genetics; transmission.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Ferrer I., Lopez-Gonzalez I., Carmona M., Arregui L., Dalfo E., Torrejon-Escribano B., Diehl R., Kovacs G.G. Glial and neuronal tau pathology in tauopathies: Characterization of disease-specific phenotypes and tau pathology progression. J. Neuropathol. Exp. Neurol. 2014;73:81–97. doi: 10.1097/NEN.0000000000000030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

