Recent Developments in Transition-Metal Catalyzed Direct C-H Alkenylation, Alkylation, and Alkynylation of Azoles
- PMID: 33121108
- PMCID: PMC7662665
- DOI: 10.3390/molecules25214970
Recent Developments in Transition-Metal Catalyzed Direct C-H Alkenylation, Alkylation, and Alkynylation of Azoles
Abstract
The transition metal-catalyzed C-H bond functionalization of azoles has emerged as one of the most important strategies to decorate these biologically important scaffolds. Despite significant progress in the C-H functionalization of various heteroarenes, the regioselective alkylation and alkenylation of azoles are still arduous transformations in many cases. This review covers recent advances in the direct C-H alkenylation, alkylation and alkynylation of azoles utilizing transition metal-catalysis. Moreover, the limitations of different strategies, chemoselectivity and regioselectivity issues will be discussed in this review.
Keywords: C–H activation; C–H functionalization; alkenylation; alkylation; alkynylation; azoles; transition metal-catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
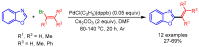
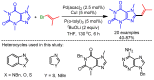
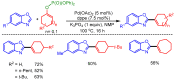
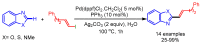

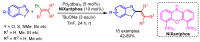

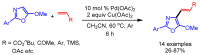
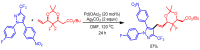
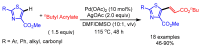
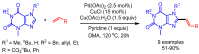
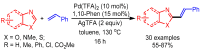
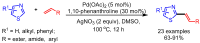

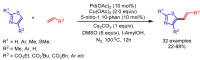
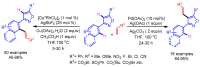
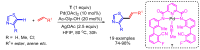


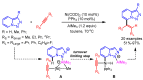
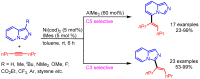

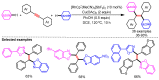
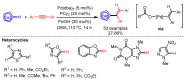
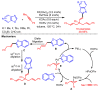
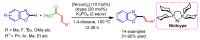

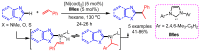


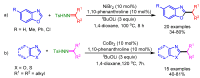
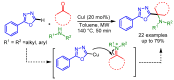

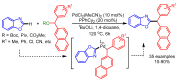
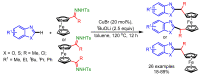
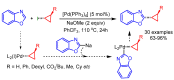
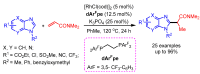
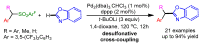
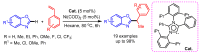
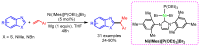

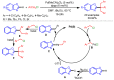

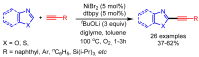
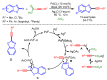
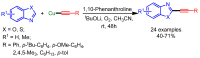

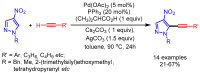
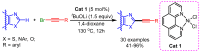
References
-
- Grimmett M.R. In: Comprehensive Heterocyclic Chemistry II. Katritzky A.R., Scriven E.F.V., editors. Volume 3. Pergamon; Oxford, UK: 1996. pp. 77–220.
-
- Woolley B.Y.D.W. From the laboratories. J. Biol. Chem. 1944;152:225–233.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

