Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology
- PMID: 33121141
- PMCID: PMC7663780
- DOI: 10.3390/ijms21217988
Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology
Abstract
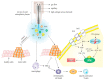
Vulvar cancer (VC) is a specific form of malignancy accounting for 5-6% of all gynaecologic malignancies. Although VC occurs most commonly in women after 60 years of age, disease incidence has risen progressively in premenopausal women in recent decades. VC demonstrates particular features requiring well-adapted therapeutic approaches to avoid potential treatment-related complications. Significant improvements in disease-free survival and overall survival rates for patients diagnosed with post-stage I disease have been achieved by implementing a combination therapy consisting of radical surgical resection, systemic chemotherapy and/or radiotherapy. Achieving local control remains challenging. However, mostly due to specific anatomical conditions, the need for comprehensive surgical reconstruction and frequent post-operative healing complications. Novel therapeutic tools better adapted to VC particularities are essential for improving individual outcomes. To this end, cold atmospheric plasma (CAP) treatment is a promising option for VC, and is particularly appropriate for the local treatment of dysplastic lesions, early intraepithelial cancer, and invasive tumours. In addition, CAP also helps reduce inflammatory complications and improve wound healing. The application of CAP may realise either directly or indirectly utilising nanoparticle technologies. CAP has demonstrated remarkable treatment benefits for several malignant conditions, and has created new medical fields, such as "plasma medicine" and "plasma oncology". This article highlights the benefits of CAP for the treatment of VC, VC pre-stages, and postsurgical wound complications. There has not yet been a published report of CAP on vulvar cancer cells, and so this review summarises the progress made in gynaecological oncology and in other cancers, and promotes an important, understudied area for future research. The paradigm shift from reactive to predictive, preventive and personalised medical approaches in overall VC management is also considered.
Keywords: cancer development; cold atmospheric plasma; gynaecological oncology; individualised profiling; patient stratification; plasma tissue interaction; predictive preventive personalised medicine (PPPM/3PM); premalignant lesions; risk factors; treatment; vulva cancer.
Conflict of interest statement
The authors and OBGY Health & Care Ltd. declare no conflict of interest.
Figures
References
-
- Graves D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012;45:3001. doi: 10.1088/0022-3727/45/26/263001. - DOI
-
- Kaushik N., Uddin N., Sim G.B., Hong Y.J., Baik K.Y., Kim C.H., Lee S.J., Kaushik N.K., Choi E.H. Responses of Solid Tumor Cells in DMEM to Reactive Oxygen Species Generated by Non-Thermal Plasma and Chemically Induced ROS Systems. Sci. Rep. 2015;5:srep08587. doi: 10.1038/srep08587. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

