Twisted Intramolecular Charge Transfer State of a "Push-Pull" Emitter
- PMID: 33121185
- PMCID: PMC7662227
- DOI: 10.3390/ijms21217999
Twisted Intramolecular Charge Transfer State of a "Push-Pull" Emitter
Abstract
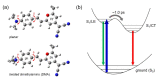
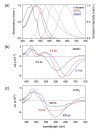
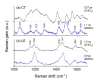
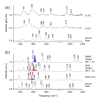
The excited state Raman spectra of 4-dicyanomethylene-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran (DCM) in the locally-excited (LE) and the intramolecular charge transfer (ICT) states have been separately measured by time-resolved stimulated Raman spectroscopy. In a polar dimethylsulfoxide solution, the ultrafast ICT of DCM with a time constant of 1.0 ps was observed in addition to the vibrational relaxation in the ICT state of 4-7 ps. On the other hand, the energy of the ICT state of DCM becomes higher than that of the LE state in a less polar chloroform solution, where the initially-photoexcited ICT state with the LE state shows the ultrafast internal conversion to the LE state with a time constant of 300 fs. The excited-state Raman spectra of the LE and ICT state of DCM showed several major vibrational modes of DCM in the LE and ICT conformer states coexisting in the excited state. Comparing to the time-dependent density functional theory simulations and the experimental results of similar push-pull type molecules, a twisted geometry of the dimethylamino group is suggested for the structure of DCM in the S1/ICT state.
Keywords: excited-state dynamics; femtosecond stimulated raman spectroscopy; intramolecular charge transfer; push-pull emitter; twisted intramolecular charge transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Intramolecular charge transfer of a push-pull chromophore with restricted internal rotation of an electron donor.Phys Chem Chem Phys. 2022 Mar 9;24(10):5794-5802. doi: 10.1039/d1cp05541k. Phys Chem Chem Phys. 2022. PMID: 35195633
-
Dynamics of ultrafast intramolecular charge transfer with 1-tert-butyl-6-cyano-1,2,3,4-tetrahydroquinoline (NTC6) in n-hexane and acetonitrile.J Phys Chem A. 2007 Dec 20;111(50):12878-90. doi: 10.1021/jp074983z. Epub 2007 Nov 23. J Phys Chem A. 2007. PMID: 18034465
-
Twisted intramolecular charge transfer of nitroaromatic push-pull chromophores.Sci Rep. 2022 Apr 21;12(1):6557. doi: 10.1038/s41598-022-10565-6. Sci Rep. 2022. PMID: 35449231 Free PMC article.
-
Synergistic Approach of Ultrafast Spectroscopy and Molecular Simulations in the Characterization of Intramolecular Charge Transfer in Push-Pull Molecules.Molecules. 2020 Jan 20;25(2):430. doi: 10.3390/molecules25020430. Molecules. 2020. PMID: 31968694 Free PMC article. Review.
-
Progress of Dicyanomethylene-4H-Pyran Derivatives in Biological Sensing Based on ICT Effect.Front Chem. 2022 May 23;10:903253. doi: 10.3389/fchem.2022.903253. eCollection 2022. Front Chem. 2022. PMID: 35677595 Free PMC article. Review.
Cited by
-
The Physical Chemistry and Chemical Physics (PCCP) Section of the International Journal of Molecular Sciences in Its Publications: The First 300 Thematic Articles in the First 3 Years.Int J Mol Sci. 2021 Dec 27;23(1):241. doi: 10.3390/ijms23010241. Int J Mol Sci. 2021. PMID: 35008667 Free PMC article.
-
Adsorption of 4-(N,N-Dimethylamino)-4'-nitrostilbene on an Amorphous Silica Glass Surface.J Phys Chem C Nanomater Interfaces. 2023 Nov 17;127(47):22964-22974. doi: 10.1021/acs.jpcc.3c05552. eCollection 2023 Nov 30. J Phys Chem C Nanomater Interfaces. 2023. PMID: 38053626 Free PMC article.
-
Intramolecular Charge Transfer of 1-Aminoanthraquinone and Ultrafast Solvation Dynamics of Dimethylsulfoxide.Int J Mol Sci. 2021 Nov 3;22(21):11926. doi: 10.3390/ijms222111926. Int J Mol Sci. 2021. PMID: 34769357 Free PMC article.
-
Ultrafast intramolecular proton transfer reactions and solvation dynamics of DMSO.Struct Dyn. 2019 Dec 12;6(6):064901. doi: 10.1063/1.5129446. eCollection 2019 Nov. Struct Dyn. 2019. PMID: 31867409 Free PMC article.
-
Intramolecular Charge Transfer of Curcumin and Solvation Dynamics of DMSO Probed by Time-Resolved Raman Spectroscopy.Int J Mol Sci. 2022 Feb 2;23(3):1727. doi: 10.3390/ijms23031727. Int J Mol Sci. 2022. PMID: 35163647 Free PMC article.
References
-
- Staykov A., Nozaki D., Yoshizawa K. Theoretical study of donor-pi-bridge-acceptor unimolecular electric rectifier. J. Phys. Chem. C. 2007;111:11699–11705. doi: 10.1021/jp072600r. - DOI
-
- Marszalek M., Nagane S., Ichake A., Humphry-Baker R., Paul V., Zakeeruddin S.M., Grätzel M. Tuning spectral properties of phenothiazine based donor–π–acceptor dyes for efficient dye-sensitized solar cells. J. Mater. Chem. 2012;22:889–894. doi: 10.1039/C1JM14024H. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

