SARS-CoV-2 vaccine candidates in rapid development
- PMID: 33121319
- PMCID: PMC7993188
- DOI: 10.1080/21645515.2020.1804777
SARS-CoV-2 vaccine candidates in rapid development
Abstract
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still spreading globally. The scientific community is attempting to procure an effective treatment and prevention strategy for COVID-19. A rising number of vaccines for COVID-19 are being developed at an unprecedented speed. Development platforms include traditional inactivated or live attenuated virus vaccines, DNA or RNA vaccines, recombinant viral vector vaccines, and protein or peptide subunit vaccines. There are 23 vaccines in the clinical evaluation stage and at least 140 candidate vaccines in preclinical evaluation. In this review, we describe research regarding basic knowledge on the virus, updates on the animal models, current landscape of vaccines in clinical evaluation and updated research results on vaccine development. Safe and effective COVID-19 vaccines require further investigation.
Keywords: COVID-19 pandemic; SARS-CoV-2; animal models; coronavirus; vaccine development.
Figures
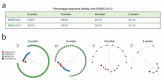


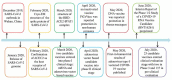
References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020. February 20;382(8):727–33. doi:10.1056/NEJMoa2001017. PubMed PMID: 31978945; PubMed Central PMCID: PMCPMC7092803. - DOI - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020. March;579(7798):270–73. doi:10.1038/s41586-020-2012-7. PubMed PMID: 32015507; PubMed Central PMCID: PMCPMC7095418. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
