Combined HIV Adolescent Prevention Study (CHAPS): comparison of HIV pre-exposure prophylaxis regimens for adolescents in sub-Saharan Africa-study protocol for a mixed-methods study including a randomised controlled trial
- PMID: 33121503
- PMCID: PMC7596950
- DOI: 10.1186/s13063-020-04760-x
Combined HIV Adolescent Prevention Study (CHAPS): comparison of HIV pre-exposure prophylaxis regimens for adolescents in sub-Saharan Africa-study protocol for a mixed-methods study including a randomised controlled trial
Abstract
Background: HIV remains a major public health issue, especially in Eastern and Southern Africa. Pre-exposure prophylaxis is highly effective when adhered to, but its effectiveness is limited by cost, user acceptability and uptake. The cost of a non-inferiority phase III trial is likely to be prohibitive, and thus, it is essential to select the best possible drug, dose and schedule in advance. The aim of this study, the Combined HIV Adolescent PrEP and Prevention Study (CHAPS), is to investigate the drug, dose and schedule of pre-exposure prophylaxis (PrEP) required for the protection against HIV and the acceptability of PrEP amongst young people in sub-Saharan Africa, and hence to inform the choice of intervention for future phase III PrEP studies and to improve strategies for PrEP implementation.
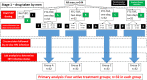
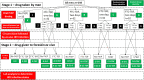
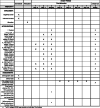
Methods: We propose a mixed-methods study amongst young people aged 13-24 years. The first component consists of qualitative research to identify the barriers and motivators towards the uptake of PrEP amongst young people in South Africa, Uganda and Zimbabwe. The second component is a randomised clinical trial (ClinicalTrials.gov NCT03986970, June 2019) using a novel ex vivo HIV challenge method to investigate the optimal PrEP treatment (FTC-TDF vs FTC-TAF), dose and schedule. We will recruit 144 amongst HIV-negative uncircumcised men aged 13-24 years from voluntary male medical circumcision clinics in two sites (South Africa and Uganda) and randomise them into one of nine arms. One group will receive no PrEP prior to surgery; the other arms will receive either FTC-TDF or FTC-TAF, over 1 or 2 days, and with the final dose given either 6 or 20 h prior to surgery. We will conduct an ex vivo HIV challenge on their resected foreskin tissue.
Discussion: This study will provide both qualitative and quantitative results to help decide the optimum drug, dose and schedule for a future phase III trial of PrEP. The study will also provide crucial information on successful strategies for providing PrEP to young people in sub-Saharan Africa.
Trial registration: ClinicalTrials.gov NCT03986970 . Registered on 14 June 2019.
Keywords: Adolescents; HIV; PrEP; South Africa; Uganda; Zimbabwe.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- UNAIDS. Fact sheet – world AIDS day 2019: UNAIDS; 2019. https://embargo.unaids.org/static/files/uploaded_files/UNAIDS_WAD2019_Fa....
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

