Leishmania-infected macrophages release extracellular vesicles that can promote lesion development
- PMID: 33122174
- PMCID: PMC7652379
- DOI: 10.26508/lsa.202000742
Leishmania-infected macrophages release extracellular vesicles that can promote lesion development
Abstract
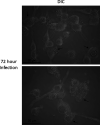
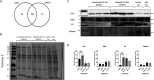
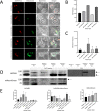
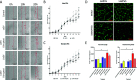
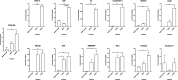
Leishmania donovani infection of macrophages results in quantitative and qualitative changes in the protein profile of extracellular vesicles (EVs) released by the infected host cells. We confirmed mass spectrometry results orthogonally by performing Western blots for several Leishmania-infected macrophage-enriched EVs (LieEVs) molecules. Several host cell proteins in LieEVs have been implicated in promoting vascular changes in other systems. We also identified 59 parasite-derived proteins in LieEVs, including a putative L. donovani homolog of mammalian vasohibins (LdVash), which in mammals promotes angiogenesis. We developed a transgenic parasite that expressed an endogenously tagged LdVash/mNeonGreen (mNG) and confirmed that LdVash/mNG is indeed expressed in infected macrophages and in LieEVs. We further observed that LieEVs induce endothelial cells to release angiogenesis promoting mediators including IL-8, G-CSF/CSF-3, and VEGF-A. In addition, LieEVs induce epithelial cell migration and tube formation by endothelial cells in surrogate angiogenesis assays. Taken together, these studies show that Leishmania infection alters the composition of EVs from infected cells and suggest that LieEVs may play a role in the promotion of vascularization of Leishmania infections.
© 2020 Gioseffi et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Bachurski D, Schuldner M, Nguyen PH, Malz A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M, et al. (2019) Extracellular vesicle measurements with nanoparticle tracking analysis: An accuracy and repeatability comparison between NanoSight NS300 and ZetaV iew. J Extracell Vesicles 8: 1596016 10.1080/20013078.2019.1596016 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
