Validation of Lung EpiCheck, a novel methylation-based blood assay, for the detection of lung cancer in European and Chinese high-risk individuals
- PMID: 33122336
- PMCID: PMC7806969
- DOI: 10.1183/13993003.02682-2020
Validation of Lung EpiCheck, a novel methylation-based blood assay, for the detection of lung cancer in European and Chinese high-risk individuals
Abstract
Aim: Lung cancer screening reduces mortality. We aim to validate the performance of Lung EpiCheck, a six-marker panel methylation-based plasma test, in the detection of lung cancer in European and Chinese samples.
Methods: A case-control European training set (n=102 lung cancer cases, n=265 controls) was used to define the panel and algorithm. Two cut-offs were selected, low cut-off (LCO) for high sensitivity and high cut-off (HCO) for high specificity. The performance was validated in case-control European and Chinese validation sets (cases/controls 179/137 and 30/15, respectively).
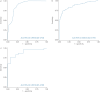
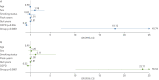
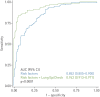
Results: The European and Chinese validation sets achieved AUCs of 0.882 and 0.899, respectively. The sensitivities/specificities with LCO were 87.2%/64.2% and 76.7%/93.3%, and with HCO they were 74.3%/90.5% and 56.7%/100.0%, respectively. Stage I nonsmall cell lung cancer (NSCLC) sensitivity in European and Chinese samples with LCO was 78.4% and 70.0% and with HCO was 62.2% and 30.0%, respectively. Small cell lung cancer (SCLC) was represented only in the European set and sensitivities with LCO and HCO were 100.0% and 93.3%, respectively. In multivariable analyses of the European validation set, the assay's ability to predict lung cancer was independent of established risk factors (age, smoking, COPD), and overall AUC was 0.942.
Conclusions: Lung EpiCheck demonstrated strong performance in lung cancer prediction in case-control European and Chinese samples, detecting high proportions of early-stage NSCLC and SCLC and significantly improving predictive accuracy when added to established risk factors. Prospective studies are required to confirm these findings. Utilising such a simple and inexpensive blood test has the potential to improve compliance and broaden access to screening for at-risk populations.
Trial registration: ClinicalTrials.gov NCT02373917.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: M. Gaga has nothing to disclose. Conflict of interest: J. Chorostowska-Wynimko reports grants, personal fees and non-financial support from Grifols, AstraZeneca, Pfizer, CSL Behring and CelonPharma, grants and personal fees from Boehringer Ingelheim, personal fees and non-financial support from MSD and BMS, personal fees from Amgen, GSK, Novartis, Chiesi, Roche and Lekam, outside the submitted work. Conflict of interest: I. Horváth reports personal fees from AstraZeneca, Novartis, CSL Behring, Boehringer Ingelheim, GSK and Berlin-Chemie, outside the submitted work. Conflict of interest: M.C. Tammemagi has served as consultant to Johnson & Johnson/Janssen, Medial EarlySign, Nucleix, bioAffinity Technologies and AstraZeneca. Conflict of interest: D. Shitrit has nothing to disclose. Conflict of interest: V.H. Eisenberg has nothing to disclose. Conflict of interest: H. Liang has nothing to disclose. Conflict of interest: D. Stav has nothing to disclose. Conflict of interest: D. Levy Faber has nothing to disclose. Conflict of interest: M. Jansen has nothing to disclose. Conflict of interest: Y. Raviv has nothing to disclose. Conflict of interest: V. Panagoulias has nothing to disclose. Conflict of interest: P. Rudzinski has nothing to disclose. Conflict of interest: G. Izbicki has nothing to disclose. Conflict of interest: O. Ronen has nothing to disclose. Conflict of interest: A. Goldhaber has nothing to disclose. Conflict of interest: R. Moalem has nothing to disclose. Conflict of interest: N. Arber has nothing to disclose. Conflict of interest: I. Haas has nothing to disclose. Conflict of interest: Q. Zhou has nothing to disclose.
Figures
Comment in
-
Biomarkers in lung cancer screening: the importance of study design.Eur Respir J. 2021 Jan 14;57(1):2004367. doi: 10.1183/13993003.04367-2020. Print 2021 Jan. Eur Respir J. 2021. PMID: 33446580 Free PMC article. No abstract available.
References
-
- International Agency for Research on Cancer GLOBOCAN2018 Global Cancer Observatory https://gco.iarc.fr. Last accessed July 2020.
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program Lung Cancer Stat Facts: Lung and Bronchus Cancer www.seer.cancer.gov/statfacts/html/lungb.html Date last accessed: December 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
