Effects of HER Family-targeting Tyrosine Kinase Inhibitors on Antibody-dependent Cell-mediated Cytotoxicity in HER2-expressing Breast Cancer
- PMID: 33122343
- PMCID: PMC7854527
- DOI: 10.1158/1078-0432.CCR-20-2007
Effects of HER Family-targeting Tyrosine Kinase Inhibitors on Antibody-dependent Cell-mediated Cytotoxicity in HER2-expressing Breast Cancer
Abstract
Purpose: Antibody-dependent cell-mediated cytotoxicity (ADCC) is one mechanism of action of the monoclonal antibody (mAb) therapies trastuzumab and pertuzumab. Tyrosine kinase inhibitors (TKIs), like lapatinib, may have added therapeutic value in combination with mAbs through enhanced ADCC activity. Using clinical data, we examined the impact of lapatinib on HER2/EGFR expression levels and natural killer (NK) cell gene signatures. We investigated the ability of three TKIs (lapatinib, afatinib, and neratinib) to alter HER2/immune-related protein levels in preclinical models of HER2-positive (HER2+) and HER2-low breast cancer, and the subsequent effects on trastuzumab/pertuzumab-mediated ADCC.
Experimental design: Preclinical studies (proliferation assays, Western blotting, high content analysis, and flow cytometry) employed HER2+ (SKBR3 and HCC1954) and HER2-low (MCF-7, T47D, CAMA-1, and CAL-51) breast cancer cell lines. NCT00524303 provided reverse phase protein array-determined protein levels of HER2/pHER2/EGFR/pEGFR. RNA-based NK cell gene signatures (CIBERSORT/MCP-counter) post-neoadjuvant anti-HER2 therapy were assessed (NCT00769470/NCT01485926). ADCC assays utilized flow cytometry-based protocols.
Results: Lapatinib significantly increased membrane HER2 levels, while afatinib and neratinib significantly decreased levels in all preclinical models. Single-agent lapatinib increased HER2 or EGFR levels in 10 of 11 (91%) tumor samples. NK cell signatures increased posttherapy (P = 0.03) and associated with trastuzumab response (P = 0.01). TKI treatment altered mAb-induced NK cell-mediated ADCC in vitro, but it did not consistently correlate with HER2 expression in HER2+ or HER2-low models. The ADCC response to trastuzumab and pertuzumab combined did not exceed either mAb alone.
Conclusions: TKIs differentially alter tumor cell phenotype which can impact NK cell-mediated response to coadministered antibody therapies. mAb-induced ADCC response is relevant when rationalizing combinations for clinical investigation.
©2020 American Association for Cancer Research.
Figures

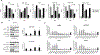


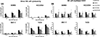
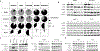
References
-
- Slamon DJ, et al., Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med, 2001. 344(11): p. 783–92. - PubMed
-
- Gullo G, et al., Long-term outcome of patients (PTS) with HER2-positive (HER2+) metastatic breast cancer (MBC) who achieved a complete response (CR) after ANTIHER2 therapy (HER2TX). Ann Oncol, 2014. 25((Suppl 4)): p. 376P, iv124-iv125.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

