Metabolic Activation and Covalent Protein Binding of Berberrubine: Insight into the Underlying Mechanism Related to Its Hepatotoxicity
- PMID: 33122887
- PMCID: PMC7588839
- DOI: 10.2147/DDDT.S274627
Metabolic Activation and Covalent Protein Binding of Berberrubine: Insight into the Underlying Mechanism Related to Its Hepatotoxicity
Abstract
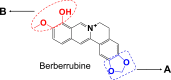
Introduction: Berberrubine (BRB), an isoquinoline alkaloid, is a major constituent of medicinal plants Coptis chinensis Franch or Phellodendron chinense Schneid. BRB exhibits various pharmacological activities, whereas exposure to BRB may cause toxicity in experimental animals.
Methods: In this study, we thoroughly investigated the liver injury induced by BRB in mice and rats. To explore the underlying mechanism, a study of the metabolic activation of BRB was conducted. Furthermore, covalent modifications of cysteine residues of proteins were observed in liver homogenate samples of animals after exposure to BRB, by application of an exhaustive proteolytic digestion method.
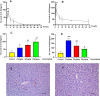
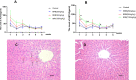
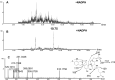
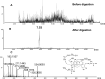
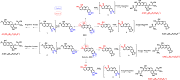
Results: It was demonstrated that BRB-induced hepatotoxicities in a time- and dose-dependent manner, based on the biochemical parameters ALT and AST. H&E stained histopathological examination showed the occurrence of obvious edema in liver of mice after intraperitoneal (i.p.) administration of BRB at a single dose of 100 mg/kg. Slight hepatotoxicity was also observed in rats given the same doses of BRB after six weeks of gavage. As a result, four GSH adducts derived from reactive metabolites of BRB were detected in microsomal incubations with BRB fortified with GSH as a trapping agent. Moreover, four cys-based adducts derived from reaction of electrophilic metabolites of BBR with proteins were found in livers.
Conclusion: These results suggested that the formation of protein adducts originating from metabolic activation of BRB could be a crucial factor of the mechanism of BRB-induced toxicities.
Keywords: berberrubine; hepatotoxicity; metabolic activation; protein modification.
© 2020 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

