Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions
- PMID: 33122905
- PMCID: PMC7591238
- DOI: 10.2147/IJN.S269630
Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells Under Hyperthermic Conditions
Abstract
Purpose: Breast cancer presents one of the highest rates of prevalence around the world. Despite this, the current breast cancer therapy is characterized by significant side effects and high risk of recurrence. The present work aimed to develop a new therapeutic strategy that may improve the current breast cancer therapy by developing a heat-sensitive liposomal nano-platform suitable to incorporate both anti-tumor betulinic acid (BA) compound and magnetic iron nanoparticles (MIONPs), in order to address both remote drug release and hyperthermia-inducing features. To address the above-mentioned biomedical purposes, the nanocarrier must possess specific features such as specific phase transition temperature, diameter below 200 nm, superparamagnetic properties and heating capacity. Moreover, the anti-tumor activity of the developed nanocarrier should significantly affect human breast adenocarcinoma cells.
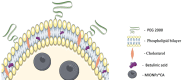
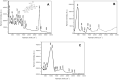
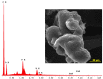
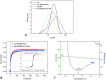
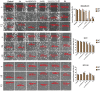
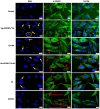
Methods: BA-loaded magnetoliposomes and corresponding controls (BA-free liposomes and liposomes containing no magnetic payload) were obtained through the thin-layer hydration method. The quality and stability of the multifunctional platforms were physico-chemically analysed by the means of RAMAN, scanning electron microscopy-EDAX, dynamic light scattering, zeta potential and DSC analysis. Besides this, the magnetic characterization of magnetoliposomes was performed in terms of superparamagnetic behaviour and heating capacity. The biological profile of the platforms and controls was screened through multiple in vitro methods, such as MTT, LDH and scratch assays, together with immunofluorescence staining. In addition, CAM assay was performed in order to assess a possible anti-angiogenic activity induced by the test samples.
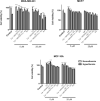
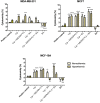
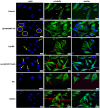
Results: The physico-chemical analysis revealed that BA-loaded magnetoliposomes present suitable characteristics for the purpose of this study, showing biocompatible phase transition temperature, a diameter of 198 nm, superparamagnetic features and heating capacity. In vitro results showed that hyperthermia induces enhanced anti-tumor activity when breast adenocarcinoma MDA-MB-231 cells were exposed to BA-loaded magnetoliposomes, while a low cytotoxic rate was exhibited by the non-tumorigenic breast epithelial MCF 10A cells. Moreover, the in ovo angiogenesis assay endorsed the efficacy of this multifunctional platform as a good strategy for breast cancer therapy, under hyperthermal conditions. Regarding the possible mechanism of action of this multifunctional nano-platform, the immunocytochemistry of the MCF7 and MDA-MB-231 breast carcinoma cells revealed a microtubule assembly modulatory activity, under hyperthermal conditions.
Conclusion: Collectively, these findings indicate that BA-loaded magnetoliposomes, under hyperthermal conditions, might serve as a promising strategy for breast adenocarcinoma treatment.
Keywords: betulinic acid; breast adenocarcinoma; hyperthermia; magnetoliposomes.
© 2020 Farcas et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- WHO. Breast cancer: prevention and control; 2020. Available from: https://www.who.int/cancer/detection/breastcancer/en/. Accessed October8, 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

