Membrane-based separation of potential emerging pollutants
- PMID: 33122962
- PMCID: PMC7592717
- DOI: 10.1016/j.seppur.2018.09.003
Membrane-based separation of potential emerging pollutants
Abstract
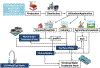
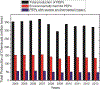
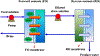
The potential emerging pollutants (PEPs) such as hazardous chemicals, toxic metals, bio-wastes, etc., pose a severe threat to human health, hygiene and ecology by way of polluting the environment and water sources. The PEPs are originated from various industrial effluent discharges including pharmaceutical, food and metal processing industries. These PEPs in contact with water may pollute the water and disturb the aquatic life. Innumerable methods have been used for the treatment of effluents and separating the toxic chemicals/metals. Of these methods, membrane-based separation processes (MBSPs) are effective over the conventional techniques for providing clean water from wastewater streams at an affordable cost with minimum energy requirement. Microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), and forward osmosis (FO) methods as well as hybrid technologies are discussed citing the published results of the past decade.
Keywords: MBSPs; PEPs; Polymers; Separation; Wastewater treatment.
Figures

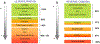


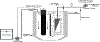
References
-
- Progress on sanitation and drinking water: 2015 update and MDG assessment https://www.wssinfo.org/fileadmin/user_upload/resources/JMP-Update-repor.... Accessed on 05-05-2017, ISBN 978 92 4 150914 5, UNICEF and World Health Organization 2015.
-
- Deblonde T, Cossu-Leguille C, Hartemann P, Emerging pollutants in wastewater: A review of the literature, Int. J. Hyg. Environ. Health 214 (2011) 442–448. - PubMed
-
- Stuart M, Lapworth D, Crane E, Hart A, Review of risk from potential emerging contaminants in UK groundwater, Sci. Total Environ. 416 (2012) 1–21. - PubMed
-
- Nasseri S, Ebrahimi S, Abtahi M, Saeedi R, Synthesis and characterization of polysulfone/graphene oxide nano-composite membranes for removal of bisphenol A from water, J. Environ. Manage 205 (2018) 174–182. - PubMed
-
- Ortego JD, Aminabhavi TM, Harlapur SF, Balundgi RH, A review of polymeric geosynthetics used in hazardous waste facilities, J. Hazard. Mater 42 (1995) 115–156.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
