Instrument-model refinement in normalized reciprocal-vector space for X-ray Laue diffraction
- PMID: 33122973
- PMCID: PMC7534537
- DOI: 10.1107/S1600576720011929
Instrument-model refinement in normalized reciprocal-vector space for X-ray Laue diffraction
Abstract
A simple yet efficient instrument-model refinement method for X-ray diffraction data is presented and discussed. The method is based on least-squares minimization of differences between respective normalized (i.e. unit length) reciprocal vectors computed for adjacent frames. The approach was primarily designed to work with synchrotron X-ray Laue diffraction data collected for small-molecule single-crystal samples. The method has been shown to work well on both simulated and experimental data. Tests performed on simulated data sets for small-molecule and protein crystals confirmed the validity of the proposed instrument-model refinement approach. Finally, examination of data sets collected at both BioCARS 14-ID-B (Advanced Photon Source) and ID09 (European Synchrotron Radiation Facility) beamlines indicated that the approach is capable of retrieving goniometer parameters (e.g. detector distance or primary X-ray beam centre) reliably, even when their initial estimates are rather inaccurate.
Keywords: Laue diffraction; X-ray diffraction; data processing; instrument models; refinement.
© International Union of Crystallography 2020.
Figures
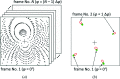
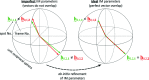
 with the same hkl indices [see e.g. Fig. 1 ▸(b)] reconstructed from spot positions, goniometer setting angles and other instrument model parameters (e.g. detector distance, primary beam position etc.). Left panel: imperfect IM parameters (the respective vectors do not overlap); right panel: ideal IM parameters (the reconstructed vectors overlap perfectly after the least-squares minimization of vector differences with respect to IM parameters).
with the same hkl indices [see e.g. Fig. 1 ▸(b)] reconstructed from spot positions, goniometer setting angles and other instrument model parameters (e.g. detector distance, primary beam position etc.). Left panel: imperfect IM parameters (the respective vectors do not overlap); right panel: ideal IM parameters (the reconstructed vectors overlap perfectly after the least-squares minimization of vector differences with respect to IM parameters).References
-
- Arvai, A. (2019). Adxv – A Program to Display X-ray Diffraction Images, https://www.scripps.edu/tainer/arvai/adxv.html.
-
- Borgstahl, G. E. O., Williams, D. R. & Getzoff, E. D. (1995). Biochemistry, 34, 6278–6287. - PubMed
-
- Campbell, J. W. (1995). J. Appl. Cryst. 28, 228–236.
Grants and funding
LinkOut - more resources
Full Text Sources
