Novel Porcine Retina Cultivation Techniques Provide Improved Photoreceptor Preservation
- PMID: 33122987
- PMCID: PMC7573241
- DOI: 10.3389/fnins.2020.556700
Novel Porcine Retina Cultivation Techniques Provide Improved Photoreceptor Preservation
Abstract
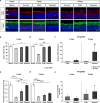
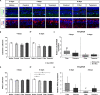
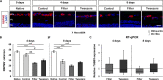
Age-related macular degeneration (AMD) is the leading cause of blindness in industrialized countries among people over 60 years. It has multiple triggers and risk factors, but despite intense research efforts, its pathomechanisms are currently not completely understood. AMD pathogenesis is characterized by soft drusen in Bruch's membrane and involves the retinal pigment epithelium-Bruch's membrane-choroid complex and adjacent structures, like photoreceptors. This study explores the potential of novel cultivation techniques to preserve photoreceptors in retinal explants to gain better insights in AMD pathology. The porcine retina explants were cultured for 4 and 8 days using three different explantation techniques, namely, control (photoreceptors facing down, touching the filter), filter (photoreceptors facing up, turned sample using a filter), and tweezers (photoreceptors facing up, turned sample using tweezers). Optical coherence tomography revealed that the tweezers method had the best capacity to limit thinning of the retinal explants. Both novel methods displayed advantages in maintaining outer segment thickness. Additionally, immunofluorescence evaluation revealed a better preservation of opsin+ cells and rhodopsin signal intensity in both novel methods, especially the tweezers method. Furthermore, RT-qPCR analysis demonstrated an upregulation of OPSIN and RHODOPSIN mRNA expression in tweezers samples at 8 days. Amacrine and bipolar cell numbers were not altered at day 4 of cultivation, while cultivation until 8 days led to reduced bipolar cell numbers. At 4 days, CALRETININ mRNA was upregulated in filter samples, but protein kinase C alpha expression was downregulated. Retinal ganglion cells were diminished in both novel techniques due to a direct physical contact with the insert. Remarkably, no difference in TUBB3 mRNA expression was detected among the techniques. Nevertheless, both novel methods exhibited an improved retention of photoreceptor cells. In conclusion, the tweezers technique was the most promising one. Due to the high homology of the porcine to the human retina, it provides a reasonable alternative to in vivo rodent models. Consequently, an adapted coculture system based on the current findings may serve as an ex vivo model suitable to analyze AMD pathomechanisms and novel therapeutic approaches.
Keywords: age-related macular degeneration; opsin; optical coherence tomography; organotypic retina culture; photoreceptor; porcine; rhodopsin.
Copyright © 2020 Wagner, Reinehr, Gammel, Greulich, Hurst, Dick, Schnichels and Joachim.
Figures



Similar articles
-
Impact of Primary RPE Cells in a Porcine Organotypic Co-Cultivation Model.Biomolecules. 2022 Jul 16;12(7):990. doi: 10.3390/biom12070990. Biomolecules. 2022. PMID: 35883547 Free PMC article.
-
Drusen-associated degeneration in the retina.Invest Ophthalmol Vis Sci. 2003 Oct;44(10):4481-8. doi: 10.1167/iovs.03-0436. Invest Ophthalmol Vis Sci. 2003. PMID: 14507896
-
Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes.Surv Ophthalmol. 1999 Oct;44 Suppl 1:S10-32. doi: 10.1016/s0039-6257(99)00086-7. Surv Ophthalmol. 1999. PMID: 10548114
-
Stem cell based therapies for age-related macular degeneration: The promises and the challenges.Prog Retin Eye Res. 2015 Sep;48:1-39. doi: 10.1016/j.preteyeres.2015.06.004. Epub 2015 Jun 23. Prog Retin Eye Res. 2015. PMID: 26113213 Review.
-
Anaphylatoxin Signaling in Retinal Pigment and Choroidal Endothelial Cells: Characteristics and Relevance to Age-Related Macular Degeneration.Adv Exp Med Biol. 2018;1074:45-51. doi: 10.1007/978-3-319-75402-4_6. Adv Exp Med Biol. 2018. PMID: 29721926 Review.
Cited by
-
Complement Factor H Loss in RPE Cells Causes Retinal Degeneration in a Human RPE-Porcine Retinal Explant Co-Culture Model.Biomolecules. 2021 Nov 3;11(11):1621. doi: 10.3390/biom11111621. Biomolecules. 2021. PMID: 34827622 Free PMC article.
-
Hypoxic Processes Induce Complement Activation via Classical Pathway in Porcine Neuroretinas.Cells. 2021 Dec 18;10(12):3575. doi: 10.3390/cells10123575. Cells. 2021. PMID: 34944083 Free PMC article.
-
Co-cultivation of primary porcine RPE cells and neuroretina induces inflammation: a potential inflammatory AMD-model.Sci Rep. 2023 Nov 7;13(1):19345. doi: 10.1038/s41598-023-46029-8. Sci Rep. 2023. PMID: 37935821 Free PMC article.
-
Impact of Primary RPE Cells in a Porcine Organotypic Co-Cultivation Model.Biomolecules. 2022 Jul 16;12(7):990. doi: 10.3390/biom12070990. Biomolecules. 2022. PMID: 35883547 Free PMC article.
-
Retinal debris triggers cytotoxic damage in cocultivated primary porcine RPE cells.Front Neurosci. 2024 Jul 24;18:1401571. doi: 10.3389/fnins.2024.1401571. eCollection 2024. Front Neurosci. 2024. PMID: 39114482 Free PMC article.
References
-
- Bermond K., Wobbe C., Tarau I. S., Heintzmann R., Hillenkamp J., Curcio C. A., et al. (2020). Autofluorescent granules of the human retinal pigment epithelium: phenotypes, intracellular distribution, and age-related topography. Invest. Ophthalmol. Vis. Sci. 61:35. 10.1167/iovs.61.5.35 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

