Comparison of Muscle MEPs From Transcranial Magnetic and Electrical Stimulation and Appearance of Reflexes in Horses
- PMID: 33122992
- PMCID: PMC7571265
- DOI: 10.3389/fnins.2020.570372
Comparison of Muscle MEPs From Transcranial Magnetic and Electrical Stimulation and Appearance of Reflexes in Horses
Abstract
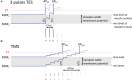
Introduction: Transcranial electrical (TES) and magnetic stimulation (TMS) are both used for assessment of the motor function of the spinal cord in horses. Muscular motor evoked potentials (mMEP) were compared intra-individually for both techniques in five healthy horses. mMEPs were measured twice at increasing stimulation intensity steps over the extensor carpi radialis (ECR), tibialis cranialis (TC), and caninus muscles. Significance was set at p < 0.05. To support the hypothesis that both techniques induce extracranially elicited mMEPs, literature was also reviewed.
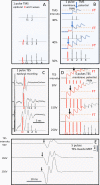
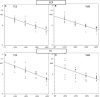
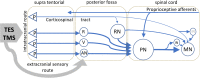
Results: Both techniques show the presence of late mMEPs below the transcranial threshold appearing as extracranially elicited startle responses. The occurrence of these late mMEPs is especially important for interpretation of TMS tracings when coil misalignment can have an additional influence. Mean transcranial motor latency times (MLT; synaptic delays included) and conduction velocities (CV) of the ECR and TC were significantly different between both techniques: respectively, 4.2 and 5.5 ms (MLT TMS --MLT TES ), and -7.7 and -9.9 m/s (CV TMS -CV TES ). TMS and TES show intensity-dependent latency decreases of, respectively, -2.6 (ECR) and -2.7 ms (TC)/30% magnetic intensity and -2.6 (ECR) and -3.2 (TC) ms/30V. When compared to TMS, TES shows the lowest coefficients of variation and highest reproducibility and accuracy for MLTs. This is ascribed to the fact that TES activates a lower number of cascaded interneurons, allows for multipulse stimulation, has an absence of coil repositioning errors, and has less sensitivity for varying degrees of background muscle tonus. Real axonal conduction times and conduction velocities are most closely approximated by TES.
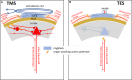
Conclusion: Both intracranial and extracranial mMEPs inevitably carry characteristics of brainstem reflexes. To avoid false interpretations, transcranial mMEPs can be identified by a stepwise latency shortening of 15-20 ms when exceeding the transcranial motor threshold at increasing stimulation intensities. A ring block around the vertex is advised to reduce interference by extracranial mMEPs. mMEPs reflect the functional integrity of the route along the brainstem nuclei, extrapyramidal motor tracts, propriospinal neurons, and motoneurons. The corticospinal tract appears subordinate in horses. TMS and TES are interchangeable for assessing the functional integrity of motor functions of the spinal cord. However, TES reveals significantly shorter MLTs, higher conduction velocities, and better reproducibility.
Keywords: TES; TMS; horses; motor potentials; neurology; startle reflex; transcranial stimulation.
Copyright © 2020 Journée, Journée, Berends, Reed, de Bruijn and Delesalle.
Figures



References
-
- Amassian V. E., Eberle L., Maccabee P. J., Cracco R. Q. (1992). Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr. Clin. Neurophysiol. 85 291–301. 10.1016/0168-5597(92)90105-k - DOI - PubMed
LinkOut - more resources
Full Text Sources

