Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2
- PMID: 33122999
- PMCID: PMC7573115
- DOI: 10.3389/fnana.2020.596439
Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV2
Abstract
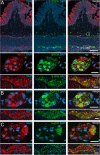
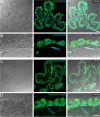
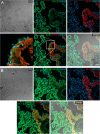
Evidence is mounting that the novel corona virus SARS-CoV2 inflicts neurological symptoms in a subgroup of COVID-19 patients. While plenty of theories on the route of neuroinvasion have been proposed, little histological evidence has been presented supporting any of these hypotheses. Therefore, we carried out immunostainings for ACE2 and TMPRSS2, two proteinases crucial for the entry of SARS-CoV2 into host cells, in the human enteric nervous system (ENS), as well as in the choroid plexus of the lateral ventricles. Both of these sites are important, yet often neglected entry gates to the nervous system. We found that ACE2 and TMPRSS2 are expressed by enteric neurons and glial cells of the small and large intestine, as well as choroid plexus epithelial cells, indicating that these cells meet the molecular requirements for viral entry. Together, our results are fundamental histological evidence substantiating current theories of neuroinvasion by SARS-CoV2.
Keywords: SARS-CoV2; choroid plexus; enteric nervous system; neuro-COVID; neuroinvasion.
Copyright © 2020 Deffner, Scharr, Klingenstein, Klingenstein, Milazzo, Scherer, Wagner, Hirt, Mack and Neckel.
Figures


References
-
- Bilinska K., Jakubowska P., Von Bartheld C. S., Butowt R. (2020). Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 11 1555–1562. 10.1021/acschemneuro.0c00210 - DOI - PMC - PubMed
-
- Bösel J., Berlit P. (2020). Neurological effects of COVID-19. DGNeurologie 3 277–284. 10.1007/s42451-020-00191-9.) - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

