Onion Bulb Extract Downregulates EGFR/ERK1/2/AKT Signaling Pathway and Synergizes With Steroids to Inhibit Allergic Inflammation
- PMID: 33123005
- PMCID: PMC7567342
- DOI: 10.3389/fphar.2020.551683
Onion Bulb Extract Downregulates EGFR/ERK1/2/AKT Signaling Pathway and Synergizes With Steroids to Inhibit Allergic Inflammation
Abstract
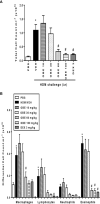
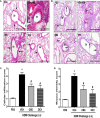
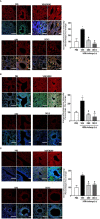
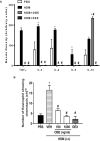
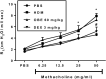
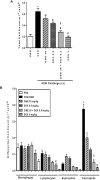
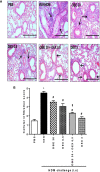
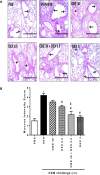
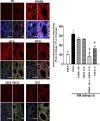
The treatment of allergic diseases, such as asthma, with both conventional and novel therapies presents a challenge both in terms of optimal effect and cost. On the other hand, traditional therapies utilizing natural products such as onion have been in use for centuries with demonstrated efficacy and safety but without much knowledge of their mechanims of action. In this study, we investigated if the anti-inflammatory effects of onion bulb extract (OBE) are mediated via the modulation of the EGFR/ERK1/2/AKT signaling pathway, and whether OBE can synergise with steroids to produce greater anti-inflammatory actions. Treatment with OBE inhibited the house dust mite (HDM)-induced increased phosphorylation of EGFR, ERK1/2 and AKT which resulted in the inhibition of HDM-induced increase in airway cellular influx, perivascular and peribronchial inflammation, goblet cell hyper/metaplasia, and also inhibited ex vivo eosinophil chemotaxis. Moreover, treatment with a combination of a low dose OBE and low dose dexamethasone resulted in a significant inhibition of the HDM-induced cellular influx, perivascular and peribronchial inflammation, goblet cell hyper/metaplasia, and increased the pERK1/2 levels, whereas neither treatment, when given alone, had any discernible effects. This study therefore shows that inhibition of the EGFR/ERK1/2/AKT-dependent signaling pathway is one of the key mechanisms by which OBE can mediate its anti-inflammatory effects in diseases such as asthma. Importantly, this study also demonstrates that combining OBE with steroids results in significantly enhanced anti-inflammatory effects. This action may have important potential implications for future asthma therapy.
Keywords: asthma; onion (Allium cepa Hysam); signalling; steroids; synergisctic effects.
Copyright © 2020 El-Hashim, Khajah, Orabi, Balakrishnan, Sary and Abdelali.
Figures


Similar articles
-
Treatment with onion bulb extract both prevents and reverses allergic inflammation in a murine model of asthma.Pharm Biol. 2024 Dec;62(1):326-340. doi: 10.1080/13880209.2024.2335187. Epub 2024 Apr 8. Pharm Biol. 2024. PMID: 38584568 Free PMC article.
-
Ang-(1-7)/ MAS1 receptor axis inhibits allergic airway inflammation via blockade of Src-mediated EGFR transactivation in a murine model of asthma.PLoS One. 2019 Nov 1;14(11):e0224163. doi: 10.1371/journal.pone.0224163. eCollection 2019. PLoS One. 2019. PMID: 31675376 Free PMC article.
-
Src-dependent EGFR transactivation regulates lung inflammation via downstream signaling involving ERK1/2, PI3Kδ/Akt and NFκB induction in a murine asthma model.Sci Rep. 2017 Aug 30;7(1):9919. doi: 10.1038/s41598-017-09349-0. Sci Rep. 2017. PMID: 28855674 Free PMC article.
-
Onion bulb extract reduces colitis severity in mice via modulation of colonic inflammatory pathways and the apoptotic machinery.J Ethnopharmacol. 2019 Sep 15;241:112008. doi: 10.1016/j.jep.2019.112008. Epub 2019 May 31. J Ethnopharmacol. 2019. PMID: 31158441
-
Epidermal growth factor receptor signalling regulates granulocyte-macrophage colony-stimulating factor production by airway epithelial cells and established allergic airway disease.Clin Exp Allergy. 2016 Feb;46(2):317-28. doi: 10.1111/cea.12612. Clin Exp Allergy. 2016. PMID: 26263242 Free PMC article.
Cited by
-
Effects of Lidocaine-Derived Organic Compounds on Eosinophil Activation and Survival.Molecules. 2023 Jul 27;28(15):5696. doi: 10.3390/molecules28155696. Molecules. 2023. PMID: 37570665 Free PMC article.
-
Treatment with onion bulb extract both prevents and reverses allergic inflammation in a murine model of asthma.Pharm Biol. 2024 Dec;62(1):326-340. doi: 10.1080/13880209.2024.2335187. Epub 2024 Apr 8. Pharm Biol. 2024. PMID: 38584568 Free PMC article.
-
Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.).Front Nutr. 2021 Jul 22;8:669805. doi: 10.3389/fnut.2021.669805. eCollection 2021. Front Nutr. 2021. PMID: 34368207 Free PMC article. Review.
-
Bioactivity-guided fractionation of Withania somnifera (L.) Dunal roots extract: evaluation of the anti-progressive potential on human Kelly neuroblastoma cell line.BMC Complement Med Ther. 2025 Jul 16;25(1):267. doi: 10.1186/s12906-025-05018-2. BMC Complement Med Ther. 2025. PMID: 40670996 Free PMC article.
References
-
- Abdel-Lateef E., Rabia I., Abdel-Gawad M., El-Sayed M. (2018). In vitro antischistosomal activity of Allium cepa L. (red onion) extracts and identification of the essential oil composition by GC-MS. J. Microbiol. Biotechnol. Food Sci. 7, 421–425. 10.15414/jmbfs.2018.7.4.421-425 - DOI
-
- Acciani T. H., Suzuki T., Trapnell B. C., Le Cras T. D. (2016). Epidermal growth factor receptor signalling regulates granulocyte-macrophage colony-stimulating factor production by airway epithelial cells and established allergic airway disease. Clin. Exp. Allergy 46 (2), 317–328. 10.1111/cea.12612 - DOI - PMC - PubMed
-
- Amishima M., Munakata M., Nasuhara Y., Sato A., Takahashi T., Homma Y., et al. (1998). Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am. J. Respir. Crit. Care Med. 157 (6 Pt 1), 1907–1912. 10.1164/ajrccm.157.6.9609040 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

