The Impact of Adipose Tissue-Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer
- PMID: 33123088
- PMCID: PMC7573351
- DOI: 10.3389/fendo.2020.563816
The Impact of Adipose Tissue-Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer
Abstract
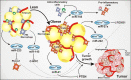
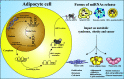
Obesity is a multifactorial and complex condition that is characterized by abnormal and excessive white adipose tissue accumulation, which can lead to the development of metabolic diseases, such as type 2 diabetes mellitus, nonalcoholic fatty liver disease, cardiovascular diseases, and several types of cancer. Obesity is characterized by excessive adipose tissue accumulation and associated with alterations in immunity, displaying a chronic low-grade inflammation profile. Adipose tissue is a dynamic and complex endocrine organ composed not only by adipocytes, but several immunological cells, which can secrete hormones, cytokines and many other factors capable of regulating metabolic homeostasis and several critical biological pathways. Remarkably, adipose tissue is a major source of circulating microRNAs (miRNAs), recently described as a novel form of adipokines. Several adipose tissue-derived miRNAs are deeply associated with adipocytes differentiation and have been identified with an essential role in obesity-associated inflammation, insulin resistance, and tumor microenvironment. During obesity, adipose tissue can completely change the profile of the secreted miRNAs, influencing circulating miRNAs and impacting the development of different pathological conditions, such as obesity, metabolic syndrome, and cancer. In this review, we discuss how miRNAs can act as epigenetic regulators affecting adipogenesis, adipocyte differentiation, lipid metabolism, browning of the white adipose tissue, glucose homeostasis, and insulin resistance, impacting deeply obesity and metabolic diseases. Moreover, we characterize how miRNAs can often act as oncogenic and tumor suppressor molecules, significantly modulating cancer establishment and progression. Furthermore, we highlight in this manuscript how adipose tissue-derived miRNAs can function as important new therapeutic targets.
Keywords: miRNA; adipose tissue; cancer; metabolic syndrome; obesity.
Copyright © 2020 Heyn, Corrêa and Magalhães.
Figures
Similar articles
-
Adipocyte, Immune Cells, and miRNA Crosstalk: A Novel Regulator of Metabolic Dysfunction and Obesity.Cells. 2021 Apr 24;10(5):1004. doi: 10.3390/cells10051004. Cells. 2021. PMID: 33923175 Free PMC article. Review.
-
MicroRNAs in adipose tissue: their role in adipogenesis and obesity.Int J Obes (Lond). 2013 Mar;37(3):325-32. doi: 10.1038/ijo.2012.59. Epub 2012 Apr 24. Int J Obes (Lond). 2013. PMID: 22531086 Review.
-
MicroRNAs and other non-coding RNAs in adipose tissue and obesity: emerging roles as biomarkers and therapeutic targets.Clin Sci (Lond). 2019 Jan 3;133(1):23-40. doi: 10.1042/CS20180890. Print 2019 Jan 15. Clin Sci (Lond). 2019. PMID: 30606812 Review.
-
The clinical potential of circulating microRNAs in obesity.Nat Rev Endocrinol. 2019 Dec;15(12):731-743. doi: 10.1038/s41574-019-0260-0. Epub 2019 Oct 14. Nat Rev Endocrinol. 2019. PMID: 31611648 Review.
-
MicroRNA regulatory networks in human adipose tissue and obesity.Nat Rev Endocrinol. 2015 May;11(5):276-88. doi: 10.1038/nrendo.2015.25. Epub 2015 Mar 3. Nat Rev Endocrinol. 2015. PMID: 25732520 Review.
Cited by
-
MicroRNA profiling of subcutaneous adipose tissue in periparturient dairy cows at high or moderate body condition.Sci Rep. 2022 Aug 30;12(1):14748. doi: 10.1038/s41598-022-18956-5. Sci Rep. 2022. PMID: 36042230 Free PMC article.
-
Epigenetic modifications in obesity-associated diseases.MedComm (2020). 2024 Feb 24;5(2):e496. doi: 10.1002/mco2.496. eCollection 2024 Feb. MedComm (2020). 2024. PMID: 38405061 Free PMC article. Review.
-
Adiponectin Related Vascular and Cardiac Benefits in Obesity: Is There a Role for an Epigenetically Regulated Mechanism?Front Cardiovasc Med. 2021 Nov 19;8:768026. doi: 10.3389/fcvm.2021.768026. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34869683 Free PMC article.
-
Mesenchymal Stem Cells in Adipose Tissue and Extracellular Vesicles in Ovarian Cancer Patients: A Bridge toward Metastatic Diffusion or a New Therapeutic Opportunity?Cells. 2021 Aug 18;10(8):2117. doi: 10.3390/cells10082117. Cells. 2021. PMID: 34440886 Free PMC article. Review.
-
Efficacy of Fetal Wharton's Jelly Mesenchymal Stem Cells-Derived Small Extracellular Vesicles in Metabolic Syndrome.Biomolecules. 2025 Jan 1;15(1):44. doi: 10.3390/biom15010044. Biomolecules. 2025. PMID: 39858439 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

