Tackling Multidrug Resistance in Streptococci - From Novel Biotherapeutic Strategies to Nanomedicines
- PMID: 33123110
- PMCID: PMC7573253
- DOI: 10.3389/fmicb.2020.579916
Tackling Multidrug Resistance in Streptococci - From Novel Biotherapeutic Strategies to Nanomedicines
Abstract
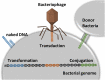
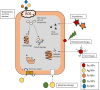
The pyogenic streptococci group includes pathogenic species for humans and other animals and has been associated with enduring morbidity and high mortality. The main reason for the treatment failure of streptococcal infections is the increased resistance to antibiotics. In recent years, infectious diseases caused by pyogenic streptococci resistant to multiple antibiotics have been raising with a significant impact to public health and veterinary industry. The rise of antibiotic-resistant streptococci has been associated to diverse mechanisms, such as efflux pumps and modifications of the antimicrobial target. Among streptococci, antibiotic resistance emerges from previously sensitive populations as result of horizontal gene transfer or chromosomal point mutations due to excessive use of antimicrobials. Streptococci strains are also recognized as biofilm producers. The increased resistance of biofilms to antibiotics among streptococci promote persistent infection, which comprise circa 80% of microbial infections in humans. Therefore, to overcome drug resistance, new strategies, including new antibacterial and antibiofilm agents, have been studied. Interestingly, the use of systems based on nanoparticles have been applied to tackle infection and reduce the emergence of drug resistance. Herein, we present a synopsis of mechanisms associated to drug resistance in (pyogenic) streptococci and discuss some innovative strategies as alternative to conventional antibiotics, such as bacteriocins, bacteriophage, and phage lysins, and metal nanoparticles. We shall provide focused discussion on the advantages and limitations of agents considering application, efficacy and safety in the context of impact to the host and evolution of bacterial resistance.
Keywords: antimicrobial resistance; bacteriocins; bacteriophage; biofilms; nanomedicine; nanoparticles; pyogenic streptococci.
Copyright © 2020 Alves-Barroco, Rivas-García, Fernandes and Baptista.
Figures

References
-
- Abdelsalam M., Asheg A., Eissa A. E. (2013). Streptococcus dysgalactiae: an emerging pathogen of fishes and mammals. Int. J. Vet. Sci. Med. 1 1–6. 10.1016/j.ijvsm.2013.04.002 - DOI
Publication types
LinkOut - more resources
Full Text Sources

