The Role of T Cells Reactive to the Cathelicidin Antimicrobial Peptide LL-37 in Acute Coronary Syndrome and Plaque Calcification
- PMID: 33123157
- PMCID: PMC7573569
- DOI: 10.3389/fimmu.2020.575577
The Role of T Cells Reactive to the Cathelicidin Antimicrobial Peptide LL-37 in Acute Coronary Syndrome and Plaque Calcification
Abstract
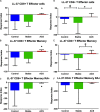
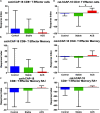
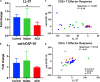
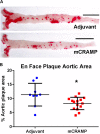
The human cationic anti-microbial peptide LL-37 is a T cell self-antigen in patients with psoriasis, who have increased risk of cardiovascular events. However, the role of LL-37 as a T cell self-antigen in the context of atherosclerosis remains unclear. The objective of this study was to test for the presence of T cells reactive to LL-37 in patients with acute coronary syndrome (ACS). Furthermore, the role of T cells reactive to LL-37 in atherosclerosis was assessed using apoE-/- mice immunized with the LL-37 mouse ortholog, mCRAMP. Peripheral blood mononuclear cells (PBMCs) from patients with ACS were stimulated with LL-37. PBMCs from stable coronary artery disease (CAD) patients or self-reported subjects served as controls. T cell memory responses were analyzed with flow cytometry. Stimulation of PBMCs with LL-37 reduced CD8+ effector T cell responses in controls and patients with stable CAD but not in ACS and was associated with reduced programmed cell death protein 1 (PDCD1) mRNA expression. For the mouse studies, donor apoE-/- mice were immunized with mCRAMP or adjuvant as controls, then T cells were isolated and adoptively transferred into recipient apoE-/- mice fed a Western diet. Recipient mice were euthanized after 5 weeks. Whole aortas and hearts were collected for analysis of atherosclerotic plaques. Spleens were collected for flow cytometric and mRNA expression analysis. Adoptive transfer experiments in apoE-/- mice showed a 28% reduction in aortic plaque area in mCRAMP T cell recipient mice (P < 0.05). Fifty six percent of adjuvant T cell recipient mice showed calcification in atherosclerotic plaques, compared to none in the mCRAMP T cell recipient mice (Fisher's exact test P = 0.003). Recipients of T cells from mice immunized with mCRAMP had increased IL-10 and IFN-γ expression in CD8+ T cells compared to controls. In conclusion, the persistence of CD8+ effector T cell response in PBMCs from patients with ACS stimulated with LL-37 suggests that LL-37-reactive T cells may be involved in the acute event. Furthermore, studies in apoE-/- mice suggest that T cells reactive to mCRAMP are functionally active in atherosclerosis and may be involved in modulating plaque calcification.
Keywords: LL-37; T cells; acute coronary syndrome; atherosclerosis; cathelicidin; mCRAMP.
Copyright © 2020 Chernomordik, Cercek, Lio, Mihailovic, Yano, Herscovici, Zhao, Zhou, Chyu, Shah and Dimayuga.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

