Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines
- PMID: 33123165
- PMCID: PMC7566192
- DOI: 10.3389/fimmu.2020.579250
Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines
Abstract
There are currently around 200 SARS-CoV-2 candidate vaccines in preclinical and clinical trials throughout the world. The various candidates employ a range of vaccine strategies including some novel approaches. Currently, the goal is to prove that they are safe and immunogenic in humans (phase 1/2 studies) with several now advancing into phase 2 and 3 trials to demonstrate efficacy and gather comprehensive data on safety. It is highly likely that many vaccines will be shown to stimulate antibody and T cell responses in healthy individuals and have an acceptable safety profile, but the key will be to confirm that they protect against COVID-19. There is much hope that SARS-CoV-2 vaccines will be rolled out to the entire world to contain the pandemic and avert its most damaging impacts. However, in all likelihood this will initially require a targeted approach toward key vulnerable groups. Collaborative efforts are underway to ensure manufacturing can occur at the unprecedented scale and speed required to immunize billions of people. Ensuring deployment also occurs equitably across the globe will be critical. Careful evaluation and ongoing surveillance for safety will be required to address theoretical concerns regarding immune enhancement seen in previous contexts. Herein, we review the current knowledge about the immune response to this novel virus as it pertains to the design of effective and safe SARS-CoV-2 vaccines and the range of novel and established approaches to vaccine development being taken. We provide details of some of the frontrunner vaccines and discuss potential issues including adverse effects, scale-up and delivery.
Keywords: Coalition for Epidemic Preparedness Innovations (CEPI); adverse events of special interest (AESI); antibody dependent enhancement (ADE); bacillus Calmette-Guérin (BCG); cell mediated immunity; innate immunity; neutralizing antibodies; spike protein.
Copyright © 2020 Flanagan, Best, Crawford, Giles, Koirala, Macartney, Russell, Teh and Wen.
Figures


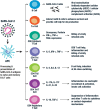
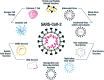
References
-
- Johns Hopkins University & Medicine, Coronavirus Resource Center COVID-19 in the USA. (2020). Available online at: http://coronavirus.jhu.edu (accessed August 26, 2020).
-
- Oke J, Heneghan C. Global Covid-19 Case Fatality Rates. (2020). Available online at: https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/ (accessed August 26, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

