Temporal changes in complement activation in haemodialysis patients with COVID-19 as a predictor of disease progression
- PMID: 33123364
- PMCID: PMC7577776
- DOI: 10.1093/ckj/sfaa192
Temporal changes in complement activation in haemodialysis patients with COVID-19 as a predictor of disease progression
Abstract
Background: Complement activation may play a pathogenic role in patients with severe coronavirus disease 2019 (COVID-19) by contributing to tissue inflammation and microvascular thrombosis.
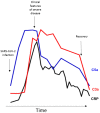
Methods: Serial samples were collected from patients receiving maintenance haemodialysis (HD). Thirty-nine patients had confirmed COVID-19 and 10 patients had no evidence of COVID-19. Plasma C5a and C3a levels were measured using enzyme-linked immunosorbent assay.
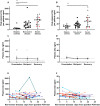
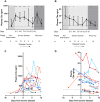
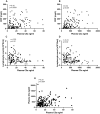
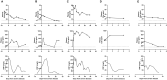
Results: We identified elevated levels of plasma C3a and C5a in HD patients with severe COVID-19 compared with controls. Serial sampling identified that C5a levels were elevated prior to clinical deterioration in patients who developed severe disease. C3a more closely mirrored both clinical and biochemical disease severity.
Conclusions: Our findings suggest that activation of complement plays a role in the pathogenesis of COVID-19, leading to endothelial injury and lung damage. C5a may be an earlier biomarker of disease severity than conventional parameters such as C-reactive protein and this warrants further investigation in dedicated biomarker studies. Our data support the testing of complement inhibition as a therapeutic strategy for patients with severe COVID-19.
Keywords: COVID-19; complement; haemodialysis.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
References
-
- World Health Organization. Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19 (8 september 2020, date last accessed)
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

