Mifepristone as a Potential Therapy to Reduce Angiogenesis and P-Glycoprotein Associated With Glioblastoma Resistance to Temozolomide
- PMID: 33123485
- PMCID: PMC7571516
- DOI: 10.3389/fonc.2020.581814
Mifepristone as a Potential Therapy to Reduce Angiogenesis and P-Glycoprotein Associated With Glioblastoma Resistance to Temozolomide
Erratum in
-
Corrigendum: Mifepristone as a Potential Therapy to Reduce Angiogenesis and P-Glycoprotein Associated With Glioblastoma Resistance to Temozolomide.Front Oncol. 2021 Mar 22;11:675806. doi: 10.3389/fonc.2021.675806. eCollection 2021. Front Oncol. 2021. PMID: 33828996 Free PMC article.
Abstract
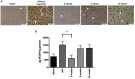
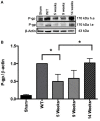
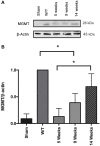
Glioblastoma, the most common primary central nervous system tumor, is characterized by extensive vascular neoformation and an area of necrosis generated by rapid proliferation. The standard treatment for this type of tumor is surgery followed by chemotherapy based on temozolomide and radiotherapy, resulting in poor patient survival. Glioblastoma is known for strong resistance to treatment, frequent recurrence and rapid progression. The aim of this study was to evaluate whether mifepristone, an antihormonal agent, can enhance the effect of temozolomide on C6 glioma cells orthotopically implanted in Wistar rats. The levels of the vascular endothelial growth factor (VEGF), and P-glycoprotein (P-gp) were examined, the former a promoter of angiogenesis that facilitates proliferation, and the latter an efflux pump transporter linked to drug resistance. After a 3-week treatment, the mifepristone/temozolomide regimen had decreased the level of VEGF and P-gp and significantly reduced tumor proliferation (detected by PET/CT images based on 18F-fluorothymidine uptake). Additionally, mifepristone proved to increase the intracerebral concentration of temozolomide. The lower level of O6-methylguanine-DNA-methyltransferase (MGMT) (related to DNA repair in tumors) previously reported for this combined treatment was herein confirmed. After the mifepristone/temozolomide treatment ended, however, the values of VEGF, P-gp, and MGMT increased and reached control levels by 14 weeks post-treatment. There was also tumor recurrence, as occurred when administering temozolomide alone. On the other hand, temozolomide led to 100% mortality within 26 days after beginning the drug treatment, while mifepristone/temozolomide enabled 70% survival 60-70 days and 30% survived over 100 days, suggesting that mifepristone could possibly act as a chemo-sensitizing agent for temozolomide.
Keywords: P-gp; angiogenesis; drug resistance; glioblastoma; mifepristone; temozolomide.
Copyright © 2020 Llaguno-Munive, León-Zetina, Vazquez-Lopez, Ramos-Godinez, Medina and Garcia-Lopez.
Figures

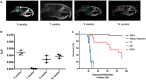
 ) and sham surgery (
) and sham surgery ( ); and in four groups with implanted glioma cancer cells, one without drug treatment (
); and in four groups with implanted glioma cancer cells, one without drug treatment ( ) and the other given temozolomide only (Tz) (
) and the other given temozolomide only (Tz) ( ), mifepristone only (Mif) (
), mifepristone only (Mif) ( ), and mifepristone/temozolomide (Mif/Tz) (
), and mifepristone/temozolomide (Mif/Tz) ( ). Each point of the graphic represents the mean ± SEM of six animals. *Significant difference (p < 0.05) between Mif/Tz and sham.
). Each point of the graphic represents the mean ± SEM of six animals. *Significant difference (p < 0.05) between Mif/Tz and sham.





References
-
- Llaguno-Munive M, Romero-Pina M, Serrano-Bello J, Medina LA, Uribe-Uribe N, Salazar AM, et al. Mifepristone overcomes tumor resistance to temozolomide associated with DNA damage repair and apoptosis in an orthotopic model of glioblastoma. Cancers. (2019) 11:16. 10.3390/cancers11010016 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

