Modulated Response of Aspergillus fumigatus and Stenotrophomonas maltophilia to Antimicrobial Agents in Polymicrobial Biofilm
- PMID: 33123497
- PMCID: PMC7573239
- DOI: 10.3389/fcimb.2020.574028
Modulated Response of Aspergillus fumigatus and Stenotrophomonas maltophilia to Antimicrobial Agents in Polymicrobial Biofilm
Abstract
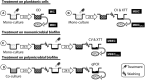
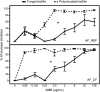
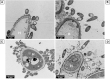
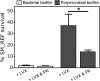
Introduction: The complexity of biofilms constitutes a therapeutic challenge and the antimicrobial susceptibility of fungal-bacterial biofilms remains poorly studied. The filamentous fungus Aspergillus fumigatus (Af) and the Gram-negative bacillus Stenotrophomonas maltophilia (Sm) can form biofilms and can be co-isolated from the airways of cystic fibrosis (CF) patients. We previously developed an in vitro biofilm model which highlighted the antibiosis effect of Sm on Af, which was dependent on the bacterial fitness. The aim of the present study was to investigate the in vitro susceptibility of Af and Sm in mono- or polymicrobial biofilms to five antimicrobial agents alone and in two-drug combinations. Methods: Af and Sm clinical reference strains and two strains from CF sputa were tested through a planktonic and biofilm approaches. Af, Sm, or Af-Sm susceptibilities to amphotericin B (AMB), itraconazole (ITC), voriconazole (VRC), levofloxacin (LVX), and rifampicin (RFN) were evaluated by conventional planktonic techniques, crystal violet, XTT, qPCR, and viable plate count. Results: Af planktonic cells and biofilms in formation were more susceptible to AMB, ITC, and VRC than Af mature biofilms. Af mature biofilms were susceptible to AMB, but not to ITC and VRC. Based on viable plate count, a lower concentration of LVX and RFN was required to reduce Sm cell numbers on biofilms in formation compared with mature biofilms. The antibiosis effect of Sm on Af growth was more pronounced for the association of CF strains that exhibited a higher fitness than the reference strains. In Af-Sm biofilms, the fungal susceptibility to AMB was increased compared with Af biofilms. In contrast, the bacterial susceptibility to LVX decreased in Af-Sm biofilms and was fungal biomass-dependent. The combination of AMB (64 μg/mL) with LVX or RFN (4 μg/mL) was efficient to impair Af and Sm growth in the polymicrobial biofilm. Conclusion: Sm increased the Af susceptibility to AMB, whereas Af protected Sm from LVX. Interactions between Af and Sm within biofilms modulate susceptibility to antimicrobial agents, opening the way to new antimicrobial strategies in CF patients.
Keywords: Aspergillus fumigatus; Stenotrophomonas maltophilia; antibacterial agent; antifungal agent; antimicrobial susceptibility; polymicrobial biofilm.
Copyright © 2020 Roisin, Melloul, Woerther, Royer, Decousser, Guillot, Dannaoui and Botterel.
Figures




References
-
- Arendrup M. C., Meletiadis J., Mouton J. W., Lagrou K., Hamal P., Guinea J., et al. (2020). Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. Available online at: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesti... (accessed April 22, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

