Nasal high-flow oxygen versus noninvasive ventilation in acute exacerbation of COPD: protocol for a randomised noninferiority clinical trial
- PMID: 33123554
- PMCID: PMC7569159
- DOI: 10.1183/23120541.00114-2020
Nasal high-flow oxygen versus noninvasive ventilation in acute exacerbation of COPD: protocol for a randomised noninferiority clinical trial
Abstract
Background: Noninvasive ventilation (NIV) is considered as the first-line treatment for acute exacerbation of COPD (AECOPD) complicated by respiratory acidosis. Recent studies demonstrate a role of nasal high-flow oxygen (NHF) in AECOPD as an alternative treatment in patients intolerant to NIV or with contraindications to it.
Aim: The study aimed to evaluate whether NHF respiratory support is noninferior compared to NIV in respect to treatment failure, defined as need for intubation or change to alternative treatment group, in patients with AECOPD and mild-to-moderate acute or acute-on-chronic hypercapnic respiratory failure.
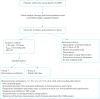
Methods: We designed a multicentre, prospective, randomised trial on patients with AECOPD, who have pH<7.35 but >7.25 and P aCO2 >45 mmHg, in whom NIV is indicated as a first-line treatment. According to power analysis, 498 participants will be required for establishing noninferiority of NHF compared to NIV. Patients will be randomly assigned to receive NIV or NHF. Treatment will be adjusted to maintain S pO2 between 88%-92% for both groups. Arterial blood gases, respiratory variables, comfort, dyspnoea score and any pulmonary or extrapulmonary complications will be assessed at baseline, before treatment initiation, and at 1, 2, 4, 6, 12, 24, 48 h, then once daily from day 3 to patient discharge, intubation or death.
Conclusion: Given the increasing number of studies demonstrating the physiological effects of NHF in COPD patients, we hypothesise that NHF respiratory support will be noninferior to NIV in patients with AECOPD and mild-to-moderate acute or acute on chronic hypercapnic respiratory failure.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: A. Papalampidou reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: E. Bibaki reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: S. Boutlas reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: I. Pantazopoulos reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: N. Athanasiou reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: M. Moylan has nothing to disclose. Conflict of interest: V. Vlachakos reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: V. Grigoropoulos reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: K. Eleftheriou reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: Z. Daniil reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: K. Gourgoulianis reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: L. Kalomenidis reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: S. Zakynthinos reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study. Conflict of interest: E. Ischaki reports nonfinancial support from Fisher & Paykel in the form of devices and consumables during the conduct of the study.
Figures
References
LinkOut - more resources
Full Text Sources
Medical